San Francisco, California (UroToday.com) Based on preclinical data from mouse models looking at radiation therapy combined with dual anti-PDL1 and anti-CTLA4 immune checkpoint blockade in cancer that showed increased T cell receptor diversity, expanded T cell clones, less T cell exhaustion, and tumor shrinkage, there has been interest in exploring combination radiation therapy and immunotherapy in metastatic renal cell carcinoma.

The authors of this abstract created the RADVAX trial of combined nivolumab and ipilimumab with SBRT preferably to 50Gy to be administered within the first three weeks of starting immunotherapy. The authors built in a safety run in of 6 patients to ensure that the combination was tolerable and did not require management of immune related adverse events. Patients were eligible for the study if they had metastatic renal cell carcinoma with a clear cell component and at least two metastatic sites, one of which was measurable. Patients were excluded if they had received prior immune checkpoint blockade, had an autoimmune disease, were on baseline prednisone doses, or were otherwise medically unfit. Importantly, SBRT to liver lesions was not allowed due to concerns for toxicity.
In total, 25 patients were enrolled at a single study and received at least 1 dose of immunotherapy and SBRT, thus being eligible for intention to treat analysis. A third of patients had their primary tumor intake, 20% of patients had received radiation as part of prior treatment, and 30% of patients had PD-L1 positive tumors.
The most commonly radiated metastatic site was the lung. All patients received the planned radiation. Five patients did not receive all planned doses of dual immune checkpoint blockade, three because of progressive disease and two because of adverse events. Safety data are summarized as follows.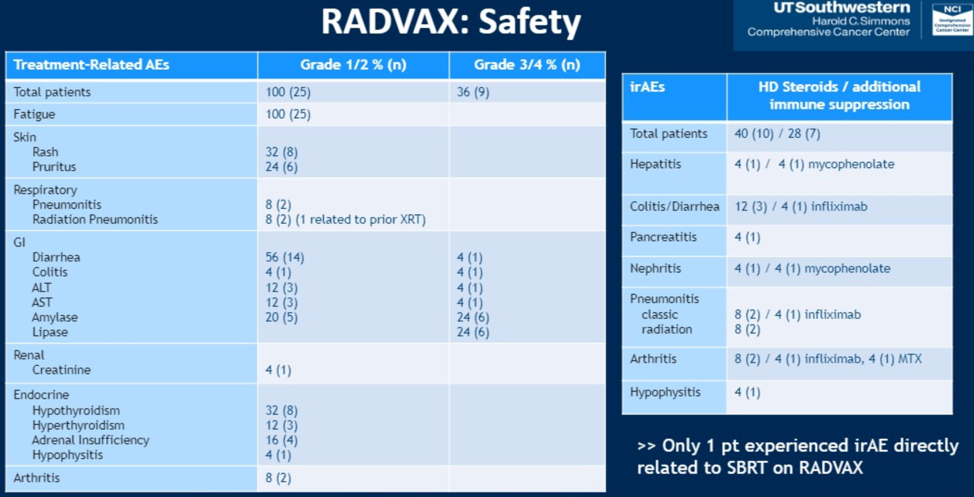
Of the 25 patient cohorts, the overall response rate was 56%, with no complete responses. The waterfall plot showing patient responses and response categories are shown below. Six of 14 patients continue to not have progressive disease. In total, the median PFS on the study was 8.2 months (95% CI 4.6-18) and the 12 month PFS rate was 36%.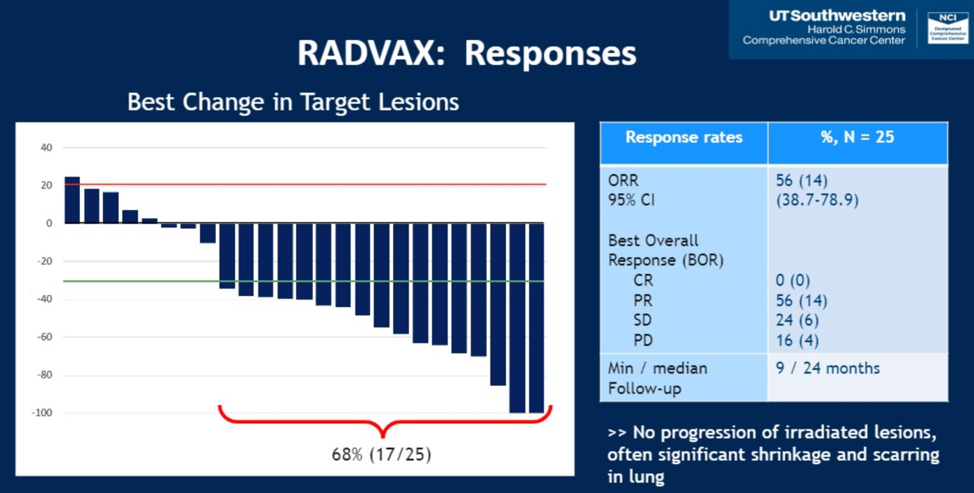
In summary, the RADVAX trial showed that SBRT combined with dual immune checkpoint inhibition is feasible with some antitumor activity. It is unclear if these findings represent a true abscopal effect versus disease control from one or the other (or both) of the treatment modalities, and further study is required to understand how to more effectively combine these treatment modalities to improve patient outcomes.
Presented by: Hans J Hammers, MD, PhD, Associate Professor, and Medical Oncologist, University of Texas Southwestern Medical Center, Dallas, TX
Written by: Alok Tewari, MD, PhD, Medical Oncology Fellow at the Dana-Farber Cancer Institute, at the 2020 Genitourinary Cancers Symposium, ASCO GU #GU20, February 13-15, 2020, San Francisco, California


