Dr. Msaouel summarized the updated phase 1B trial results of single-agent sitravatinib in metastatic clear cell RCC, which showed 94% (30/32) clinical benefit and 25% (8/32) confirmed objective response.

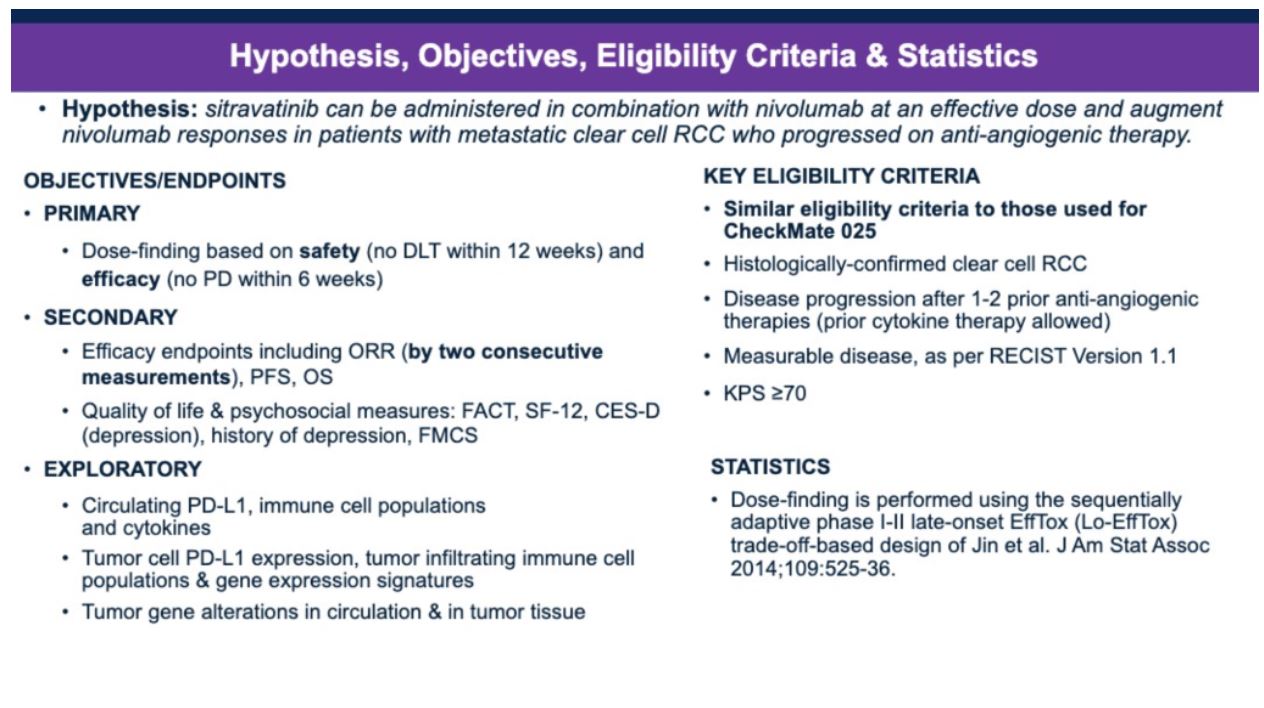
He then highlighted the objectives and study design of this combination phase I/II trial. The primary objective was to evaluate the dose based on safety (no dose-limiting toxicity within 12 weeks) and efficacy (no progression of disease within six weeks) in patients with advanced clear cell RCC that progressed on up to two prior VEGF-targeted therapies. The secondary endpoints were efficacy endpoints, including overall response rate (by two consecutive measurements), progression-free survival, overall survival, quality of life, and psychosocial measures using FACT, SF-12, CES-D (depression), history of depression, and FMCS. Eligibility criteria were similar to those used for CheckMate 025. The late-onset Bayesian EffTox design is used to account for potential late-onset immune-related toxicities and allow optimal dose-finding of sitravatinib from four dose levels (60, 80, 120, 150 mg/day) continuously based on both efficacy and toxicity.

Dr. Msaouel then summarized the results from this trial. A total of 40 patients were enrolled as of January 1st, 2020. All 40 for evaluable for safety with 38 patients evaluable for efficacy. Clinical benefit was noted in 35/38 patients (92%) with confirmed objective responses was demonstrated in 15/38 patients (ORR = 39%). Median progression-free survival with sitravatinib plus nivolumab was 10.3 months. Median follow-up was 17.7 months, median overall survival was not reached, and 30/38 patients are still alive.


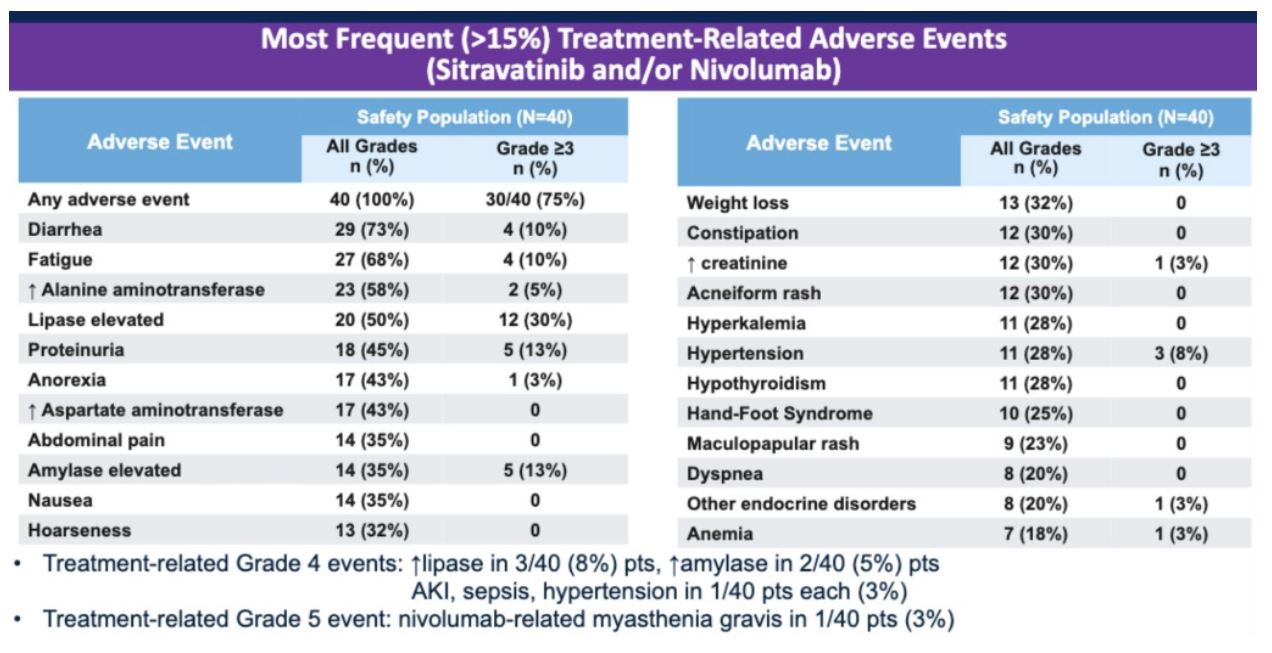
Treatment-related adverse events ≥ grade 3 was noted in 30/40 patients (75%). Toxicities requiring dose-reduction of sitravatinib were found in 21/40 patients (52.5%). 15/21 (71.4%) of these dose-limiting toxicities occurred within 12 weeks and were thus captured by the late-onset EffTox design. All three patients on the 150 mg dose of sitravatinib experienced dose-limiting toxicities within 12 weeks. There were 4 discontinuations noted due to treatment-related adverse events. The most common treatment-related adverse events included fatigue, diarrhea, hand-foot syndrome, hypertension, and elevated amylase and lipase. There was one nivolumab-related Grade 5 event (myasthenia gravis).
Dr. Msaouel then concluded his excellent summary of this phase I/II trial of sitravatinib plus nivolumab by noting that they observed higher overall response rate and longer progression-free survival than historically reported with single-agent nivolumab or single-agent cabozantinib. The combination has an acceptable toxicity profile with manageable adverse events. Sitravatinib 120mg was the most frequently selected dose by the EffTox algorithm and can be used as the acceptable toxicity dose. Analyses of patient-reported outcomes and tissue & blood correlative studies are ongoing. Sitravatinib 120mg plus nivolumab is now also being explored in other tumor types.
Presented by: Pavlos Msaouel, MD, PhD, MD Anderson Cancer Center, Houston, Texas
Written by: Abhishek Srivastava, MD, Society of Urologic Oncology Fellow, Fox Chase Cancer Center, Fox Chase Cancer Center, Philadelphia, PA, Twitter: @shekabhishek at the 2020 Genitourinary Cancers Symposium, ASCO GU #GU20, February 13-15, 2020, San Francisco, California


