Approximately 43% of patients after local therapy of localized advanced urothelial carcinoma are cured, but 57% are not cured and will have disease relapse. This is a significant number of patients for which another management path needs to be found.
There have been several trials showing that adjuvant cisplatin-based combination chemotherapy in urothelial bladder cancer improves overall survival (HR 0.77, 95% CI 0.65-0.91). Moreover, there has also been a randomized phase 3 controlled trial assessing adjuvant chemotherapy in upper tract urothelial carcinoma, showing a disease specific survival HR-0.49, 95% CI 0.31-0.761. However, there are patients who are cisplatin ineligible and patients with residual disease after neoadjuvant chemotherapy that still need therapeutic options.
Despite having a plethora of data, there are still provocative questions that need to be properly addressed in the adjuvant setting. These include whether adjuvant chemotherapy can cure patients? Can we identify patients who need treatment? Can we identify patients who benefit from treatment? Is enough attention given to biology? And what endpoints should we be looking at?
It is important we figure out exactly who needs to receive adjuvant chemotherapy. There are tissue-based parameters and blood-based parameters. There is no doubt that organ confined disease, extravesical disease, and lymph node metastatic disease have an increasing likelihood of recurrence (Figure 1).
Figure 1 – Risk of recurrence:
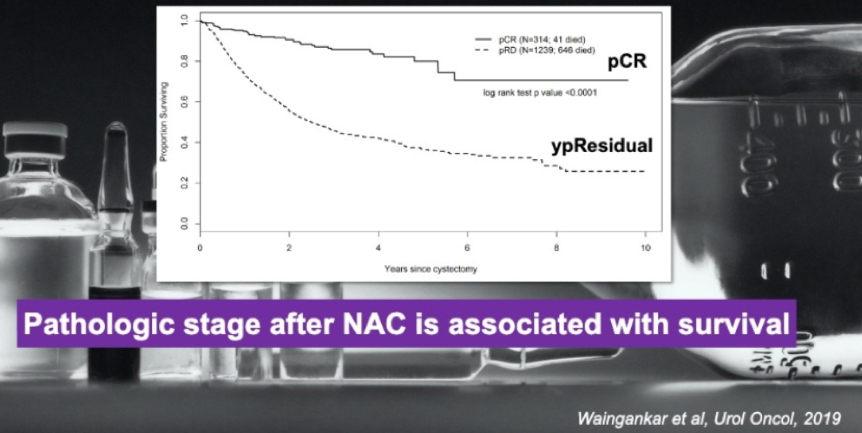
There is also evidence that pathological disease after neoadjuvant chemotherapy is associated with decreased survival (Figure 2). There is data showing that alterations in DNA damage response genes can perhaps identify patients who should receive adjuvant chemotherapy, but this needs further exploration.
Figure 2 – The association of pathological disease with survival after neoadjuvant chemotherapy:
Next, Dr. Glasky mentioned why it is critical that we understand the biology in order to choose the right patients for adjuvant chemotherapy. If a drug shows safety and activity in the metastatic setting, can it have similar outcomes in an earlier setting, and naturally moved to the perioperative setting? This is still unclear. An effective drug in the neoadjuvant setting does not automatically mean that it will be effective in the adjuvant setting as well. A recent example of this was detailed in a press release in January 24th 2020 from the IMvigor010 trial (NCT02450331), which noted that adjuvant atezolizumab did not meet its primary endpoint of disease free survival, compared to observation. This is in contrast to favorable results this drug has shown in the neoadjuvant setting, as previously shown in the ABACUS trial2.
Moreover, there is growing body of data supporting the use of neoadjuvant over adjuvant PD-1/PD-L1 blockade (Figure 3).
Figure 3 – Neoadjuvant immune checkpoint inhibitor therapy is superior to adjuvant therapy?

Concluding his talk, Dr Galsky mentioned a few important points and said that adjuvant trials in urothelial bladder cancer have faced significant challenges. CTDNA may be poised to change the adjuvant paradigm, and trial design with the right endpoints chosen are critical to decipher the right treatment path for these patients.
Presented by: Matt D. Galsky, MD, FASCO, Medical Oncologist, Professor of Medicine and of Urology, Tisch Cancer Institute and Icahn School of Medicine at Mount Sinai, New York, NY
Written by: Hanan Goldberg, MD, Urology Department, SUNY Upstate Medical University, Syracuse, NY, USA, Twitter: @GoldbergHanan, at the 2020 Genitourinary Cancers Symposium, ASCO GU #GU20, February 13-15, 2020, San Francisco, California.
References:
- Birtle AJ, Chester JD, Jones RJ, et al. Results of POUT: A phase III randomised trial of perioperative chemotherapy versus surveillance in upper tract urothelial cancer (UTUC). Journal of Clinical Oncology 2018; 36(6_suppl): 407-.
- Powles T, Rodriguez-Vida A, Duran I, et al. A phase II study investigating the safety and efficacy of neoadjuvant atezolizumab in muscle invasive bladder cancer (ABACUS). Journal of Clinical Oncology 2018; 36(15_suppl): 4506-.


