Dr. Grivas began discussing some important points on neoadjuvant chemotherapy. There is no doubt that disease and overall survival benefit from neoadjuvant chemotherapy with cisplatin-based combinations. Non-cisplatin therapy in the perioperative setting has no proven benefits in muscle-invasive bladder cancer. The chemotherapy combination of dd-MVAC may have less toxicity and shorter time to surgery. It is important to not substitute carboplatin instead of cisplatin in this setting. A total of 4 cycles of accelerated dd-MVAC or gemcitabine and cisplatin is recommended in clinical-stage N0M0. Despite these known benefits, there's a definite need for novel trials focusing on immunotherapy and biomarkers of response.
Dr. Grivas moved on to discuss the recent study by A. Necchi1 assessing the updated results of the PURE-1 study with preliminary activity of neoadjuvant pembrolizumab in patients with muscle-invasive bladder carcinoma with variant histologies. In this paper, bladder cancer variant histologies were specifically assessed and their responses to pembrolizumab. The study showed a variable response rate with a higher tumor burden and higher PD-l1 shown to be correlated to a better response rate.
In the ABACUS trial,2 two doses of atezolizumab in the neoadjuvant setting in cisplatin-ineligible patients were assessed. The pathological complete response rate was 29%. In contrast to the previously mentioned study, in this study PD-L1 expression did not appear to correlate with pathological response.
The potential explanations for the discordance between these two trials include:
- The different patient population – cisplatin-ineligible vs. cisplatin eligible
- The different agents Anti-PDL1 (atezolizumab) vs. anti-PD1 agent (pembrolizumab); two vs. three doses
- The percentage of variant histology in these trials
- The different PD-L1 expression
- The different clinical-stage distribution
- Unmeasured selection and confounding biases
- Different assays, scoring systems and bioinformatics
According to Dr. Grivas, the 3 features of an optimal biomarker include preanalytical validity, biological relevance (clinical validity) and clinical utility.
The next paper discussed addressed the early detection of metastatic relapse and monitoring of therapeutic efficacy by ultra-deep sequencing of plasma cell-free DNA in patients with urothelial bladder carcinoma3. The authors assessed 68 patients with localized MIBC urothelial carcinoma who received neoadjuvant chemotherapy and then progressed to radical cystectomy. The authors assessed available DNA from the tumor and plasma samples before and after radical cystectomy, and before and during neoadjuvant chemotherapy.
The authors assessed the prognostic value of ctDNA detection and demonstrated a survival analysis showing the probability of regression-free survival (RFS) and overall survival (OS) stratified by the ctDNA status (Figure 1).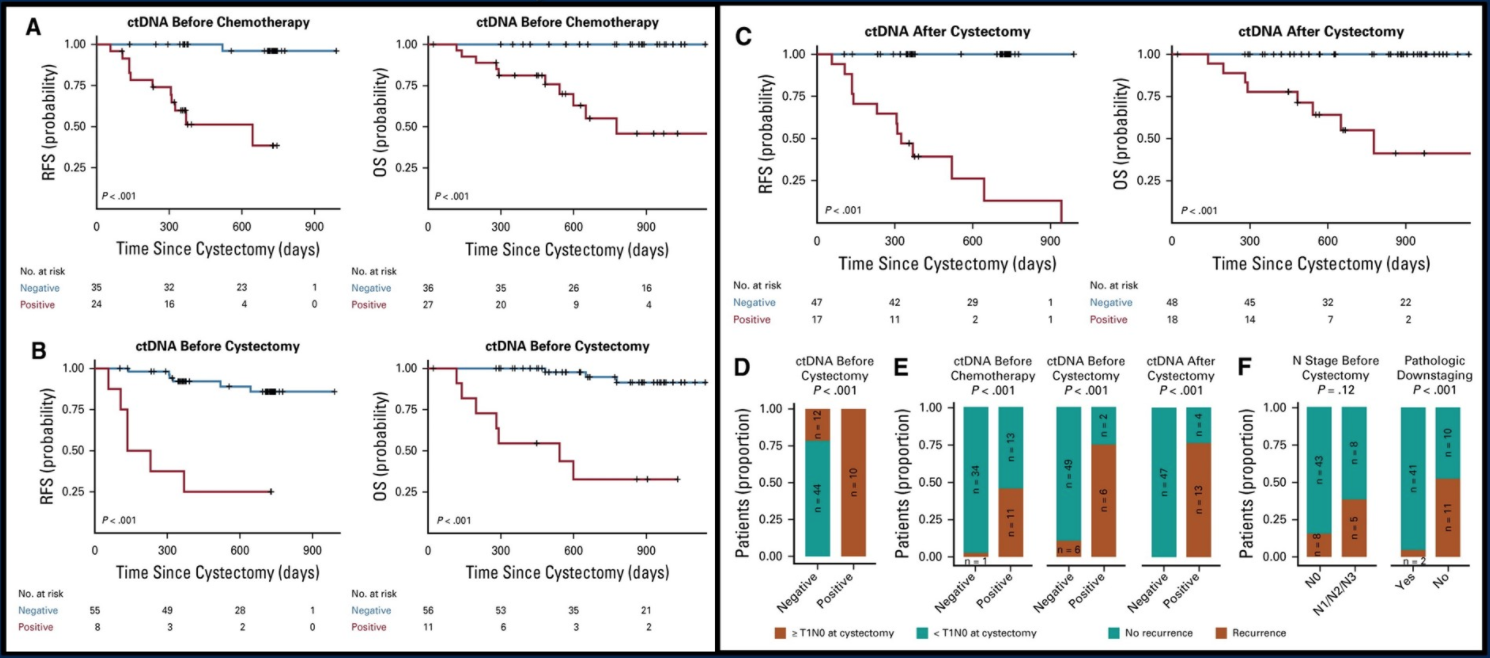
Figure 1. RFS and OS stratified by the ctDNA status
This paper raises some important questions that need to be addressed:
- Can ctDNABe used for prognostication and decision making? Probably not yet, as this needs prospective validation.
- Can ctDNA Be used as an integrated biomarker to enrich patience for adjuvant trials? possibly yes.
- The recent press release from the IMVIGOR010 trial reported no disease-free survival benefit with atezolizumab versus observation. It will be interesting to assess exploratory biomarkers in this trial and at other trials as well.
- ctDNA May serve as a quantitative surrogate of tumor burden and as liquid biopsy for NGS in target discovery and validation
The next paper discussed assessed the role of Erdafitinib in locally advanced or metastatic urothelial carcinoma4. This was an open-label Phase II study looking at locally advanced unresectable or metastatic urothelial carcinoma with prespecified FGFR alterations.
All patients had a history of progression during or after at least 1 course of chemotherapy or within 12 months after neoadjuvant chemotherapy. Patients were randomly assigned to Erdafitinib in either an intermittent or continuous regiment in the dose selection phase. The study demonstrated that erdafitinib was associated with an objective tumor response in 40% of previously treated patients, who had locally advanced and unresectable or metastatic urothelial carcinoma. Importantly, treatment-related grade 3 or higher adverse events were reported in nearly half the patients.
The last study mentioned was a pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy5. This study demonstrated an objective response rate of 44% in the analyzed patients, which was quite impressive.
Dr. Grivas concluded his excellent talk showing a suggested algorithm for the treatment of advanced urothelial carcinoma (Figure 2) and emphasized the importance of continuing to enroll patients to clinical trials throughout the disease spectrum and different treatment settings.
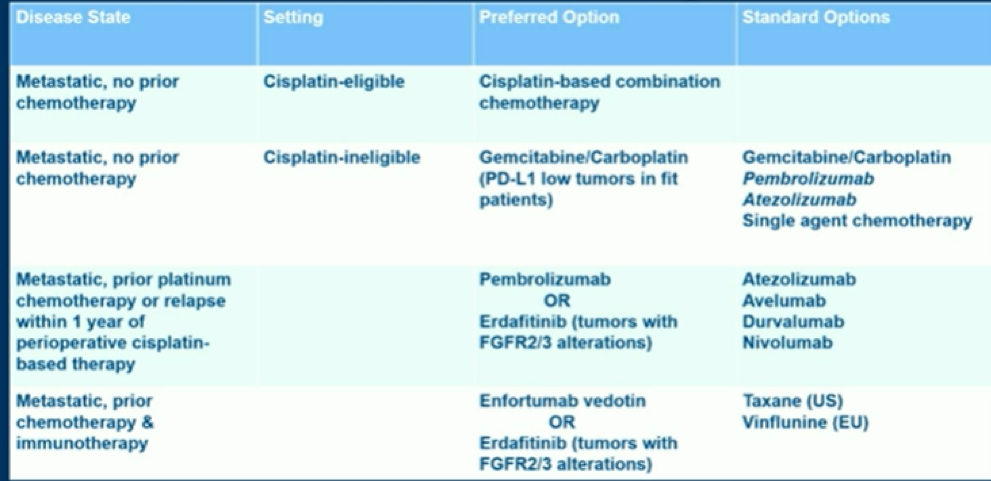
Figure 2. Advanced urothelial carcinoma treatment algorithm
Lastly, some summary points were reiterated by Dr. Grivas. He mentioned that neoadjuvant immuno-oncology seems promising but not ready for prime time yet. There is a great need for biomarkers, and it hard to perform validation due to the great variability in trials. Cell-free ctDNA appears a promising strategy but requires prospective validation of clinical utility before use in practice. Erdafitinib is getting accelerated approval for platinum-refractory advanced urothelial carcinoma with FGFR2/3 alterations (Phase III trial ongoing). Similarly, Enfortumab vedotin is also getting accelerated approval for advanced urothelial carcinoma that progressed on/after platinum-based chemotherapy and anti-PD(L)1 (Phase III trial ongoing).
We are in an exciting era with many new treatments on the horizon.
Presented by: Petros Grivas, MD, PhD, University of Washington, Seattle, Washington
Written by: Hanan Goldberg, MD, Urology Department, SUNY Upstate Medical University, Syracuse, New York, Twitter: @GoldbergHanan at the 2020 Genitourinary Cancers Symposium, ASCO GU #GU20, February 13-15, 2020, San Francisco, California
References:
- Necchi A, Raggi D, Gallina A, et al. Updated Results of PURE-01 with Preliminary Activity of Neoadjuvant Pembrolizumab in Patients with Muscle-invasive Bladder Carcinoma with Variant Histologies. Eur Urol 2019.
- Powles T, Kockx M, Rodriguez-Vida A, et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nature Medicine 2019; 25(11): 1706-14.
- Christensen E, Birkenkamp-Demtroder K, Sethi H, et al. Early Detection of Metastatic Relapse and Monitoring of Therapeutic Efficacy by Ultra-Deep Sequencing of Plasma Cell-Free DNA in Patients With Urothelial Bladder Carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2019; 37(18): 1547-57.
- Loriot Y, Necchi A, Park SH, et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. New England Journal of Medicine 2019; 381(4): 338-48.
- Rosenberg JE, O'Donnell PH, Balar AV, et al. Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma After Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2019; 37(29): 2592-600.


