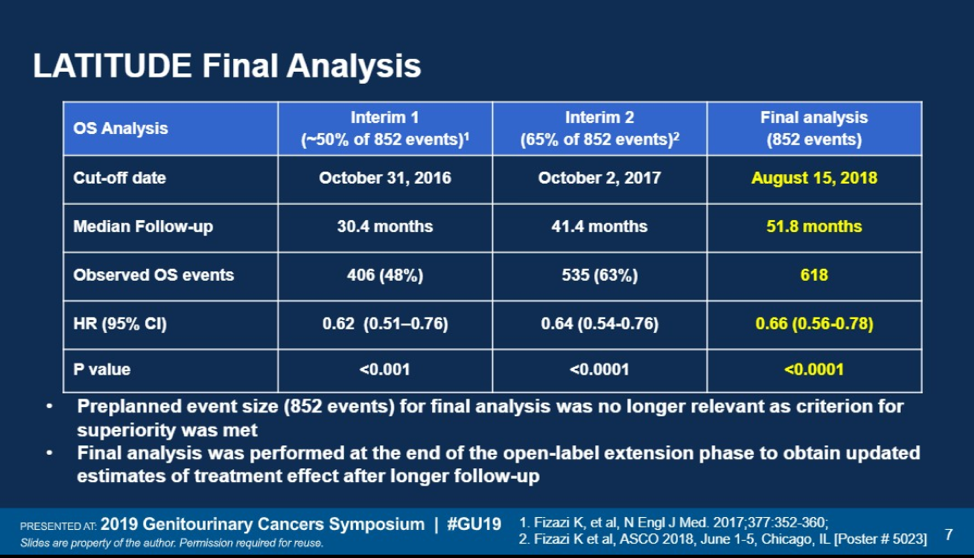
This final analysis is conducted after a median follow up of 51.8 months. A total of 618 deaths have been observed and 26.3% of patients on the abiraterone arm remain on therapy. 12% of patients have crossed over from placebo to abiraterone. At this time, abiraterone plus prednisone (AA+P) continues to show an overall survival benefit over placebo (HR: 0.7, 95% CI: 0.6-0.8; p<0.0001). The median OS was 53.3 months for patients on abiraterone and 36.5 months on placebo.
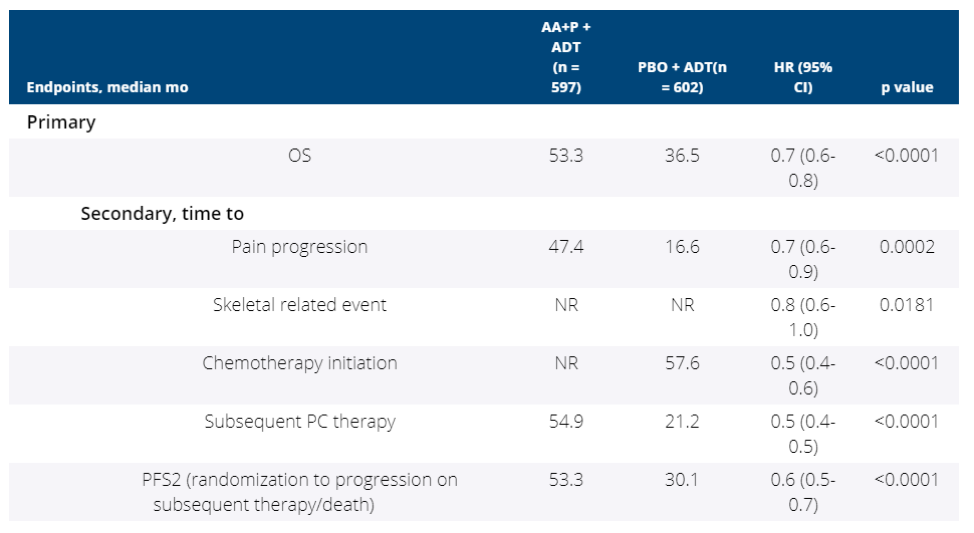
Secondary endpoint analysis shows that AA+P outperforms placebo in every category, including pain progression, skeletal related events, chemotherapy initiation, subsequent therapy, and progression to subsequent therapy or death.
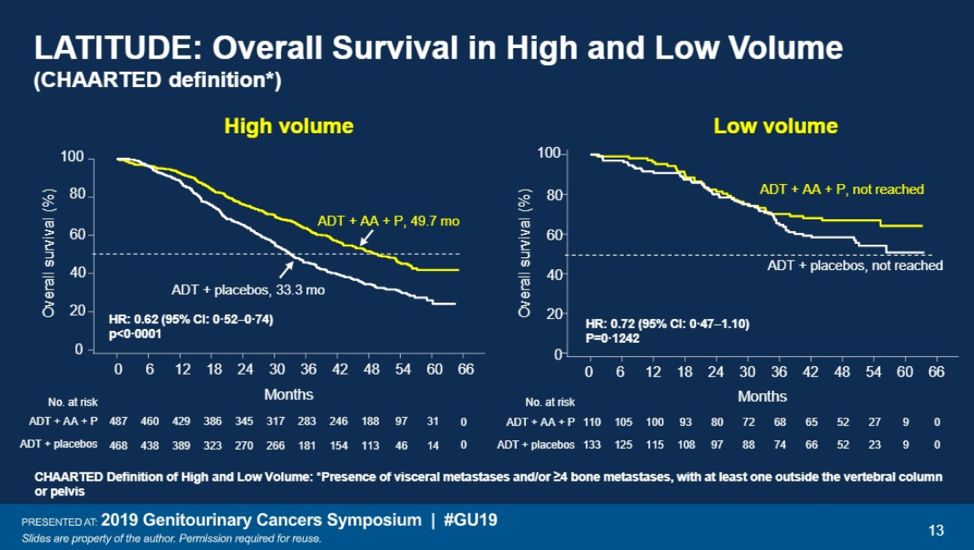
Overall survival was then analyzed based on the CHAARTED definition high (presence of visceral disease, and/or 4 or more bone metastases with at least one metastasis outside the vertebral column or pelvis) or low volume disease. Patients with high volume disease derived the most benefit with a hazard ratio of 0.62. Those with low volume disease had a HR of 0.72 with a p=0.12.
In terms of grade 3/4 adverse events, 21.9% of patients on abiraterone had hypertension, 11.7% had hypokalemia, 8.9% had hepatoxicity, and 3.9% had a cardiac disorder. Less than 1 (0.8%) of patients had fluid retention.
Based on these results, abiraterone plus prednisone remains a terrific standard of care option for patients with metastatic castration sensitive prostate cancer, significantly improving overall survival and delaying pain progression and chemotherapy progression. Patients should be closely monitored after initiating Abiraterone, with particular attention to hypertension, hepatoxicity, and hypokalemia. The choice between abiraterone and docetaxel in this disease space is complex and patient factors including performance status and cost of therapy are important to discuss. The space is also becoming more complex, as data regarding Enzalutamide look promising as well for CSPC patients (ARCHES).
Clinical Trial Information: NCT01715285
Presented by: Kim N. Chi, MD, Associate Director, Clinical Research, Senior Research Scientist, Vancouver Prostate Centre, University of British Columbia
Written by: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University, Twitter: @TheRealJasonZhu at the 2019 American Society of Clinical Oncology Genitourinary Cancers Symposium, (ASCO GU) #GU19, February 14-16, 2019 - San Francisco, CA
References:
1. Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. New England Journal of Medicine 2017;377:352-60.
2. James ND, de Bono JS, Spears MR, et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. New England Journal of Medicine 2017;377:338-51.


