While QoL metrics are often assessed in the course of these phase III studies, they do not get enough recognition and are often overshadowed by oncologic outcomes. In this study, the authors report on QoL outcomes and reporting by completing a systematic review of all phase III PCa studies published between 2012 and 2016. Naturally, this misses the many studies that have been published in the past 2 years, but this is an important start nonetheless.
These same authors had previously reported that QoL is not included among endpoints and QoL results are significantly underreported in a high proportion of recently published phase III trials in oncology. In this study, their aim was to describe QoL prevalence and heterogeneity in QoL reporting in prostate cancer (PC) phase III trials.1
As part of their overall study, the authors assessed all primary publications (P) of phase III trials evaluating anticancer drugs published between 2012 and 2016 by 11 major journals (including Annals of Oncology, JAMA, JAMA Oncology, Br J Cancer, Cancer, European J Cancer, JCO, JNCI, Lancet, Lancet Oncology). For this specific analysis, they extracted the subset of Prostate cancer (PC) trials. They then analyzed QoL inclusion among endpoints, the presence of QoL results and methodology of QoL analysis.
35 publications were identified. Of these, 21 were in castration-resistant PCa (CRPC), 9 in advanced hormone-sensitive (aHSPC) (including both metastatic and biochemical relapsed PCa), and 5 were in earlier stages of PCa). The 21 publications are summarized below:
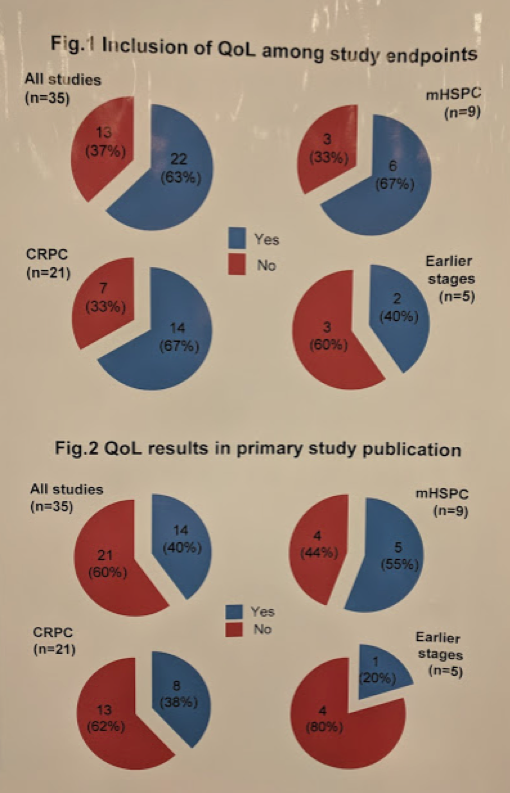
First, they assessed the presence of QoL amongst study endpoints. In 13 (37.1%) QoL was not listed among study endpoints: 7/21 (33.3%) in CRPC, 3/9 (33.3%) in aHSPC, and 3/5 (60%) in earlier stages. Off the bat, QoL is not considered important enough to assess in nearly 1/3 of all phase III studies.
Out of 22 primary publications of trials including QoL among endpoints, QoL results were not reported in 8 (36.4%). Overall, no QoL data were available in 21/35 (60%) primary P (61.9% in CRPC, 44.4% in aHSPC and 80% in earlier disease). QoL data were not available in 14/25 (56%) trials with overall survival (OS) as a primary endpoint, and in 7/10 (70%) trials with other primary endpoints. QoL data were not available in 7/16 (43.8%) trials with a positive result (25% in CRPC, 40% in aHSPC, 100% in earlier stages). So, in publications that listed QoL as an endpoint, 40% didn’t even report those endpoints!
These figures summarize the inclusion and reporting of QoL in these trials:
Last, in 18 trials with available QoL results (incl. secondary publications), the most common QoL tools used were FACT-P (11, 61.1%) and EORTC QLQ-C30 (6, 33.3%) questionnaires. When utilized, the most common methods of analysis were mean changes (6, 33.3%), mean scores over time (6, 33.3%), time to deterioration (6, 33.3%) and proportion of responders (3, 16.7%). QoL analysis was focused on the impact of toxicity in 10 cases (mostly in earlier stages) and on disease symptoms in 10 cases (mostly in CRPC).
Based on this, the authors conclude that QoL is not assessed in a high proportion of recently published phase III trials in PC (~1/3); they did note that the presence of QoL results is better in positive trials, especially in CRPC. Of studies that mentioned QoL as an endpoint, 40% didn’t report QoL outcomes. And lastly, the methodology of QoL analysis was heterogeneous in terms of the type of instruments, analysis, and presentation of results.
Significant work needs to be done on QoL assessment amongst phase III clinical trials.
Presented by: Laura Marandino, MD, Department of Oncology, University of Turin; Candiolo Cancer Institute - FPO- IRCCS, Candiolo, Italy
Written by: Thenappan Chandrasekar, MD (Clinical Instructor, Thomas Jefferson University) (twitter: @tchandra_uromd, @JEFFUrology) at the 2019 American Society of Clinical Oncology Genitourinary Cancers Symposium, (ASCO GU) #GU19, February 14-16, 2019 - San Francisco, CA
References:
- Marandino L, et al. Deficiencies in health-related quality-of-life assessment and reporting: a systematic review of oncology randomized phase III trials published between 2012 and 2016. Ann Oncol. 2018 Dec 1;29(12):2288-2295. doi: 10.1093/annonc/mdy449.


