Defects in DNA damage repair (DDR) genes have been previously associated with genomic instability, which has been shown to improve outcomes in colon cancer and bladder cancer4,5. This study examined 229 patients with metastatic clear cell carcinoma who had somatic tumor DNA testing with the MSK-IMPACT platform and outcomes were analyzed based on DDR status and treatment strategy (immunotherapy vs TKI).
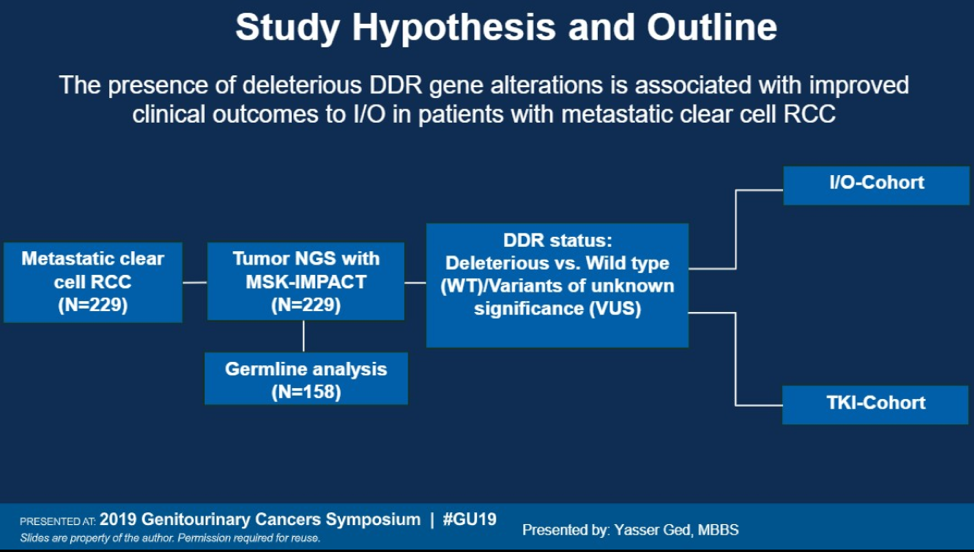
The immunotherapy cohort contained patients who had no prior exposure to immunotherapy and the TKI cohort only contained patients receiving front line TKI. No patients in this cohort were exposed to combination TKI-immunotherapy. 6 families of genes were included as part of the DDR pathway, involving damage sensors, homologous recombination, Fanconi anemia, mismatch repair, nucleotide excision repair, and base excision repair.
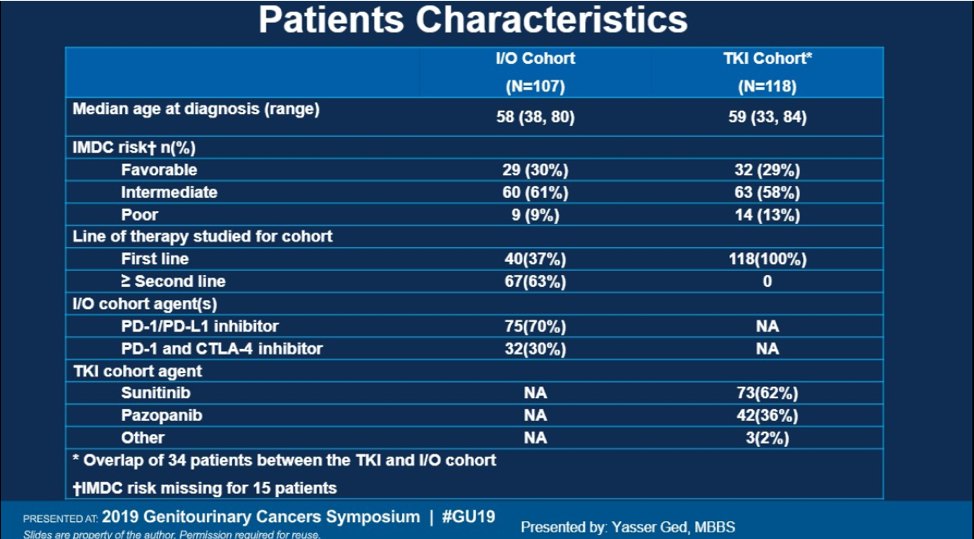
The baseline characteristics are shown below. The majority of patient were intermediate or poor risk according to IMDC criteria. In the IO arm, the majority of patients received IO in the second line (63%). In the TKI arm, sunitinib was the most commonly used TKI (62%).
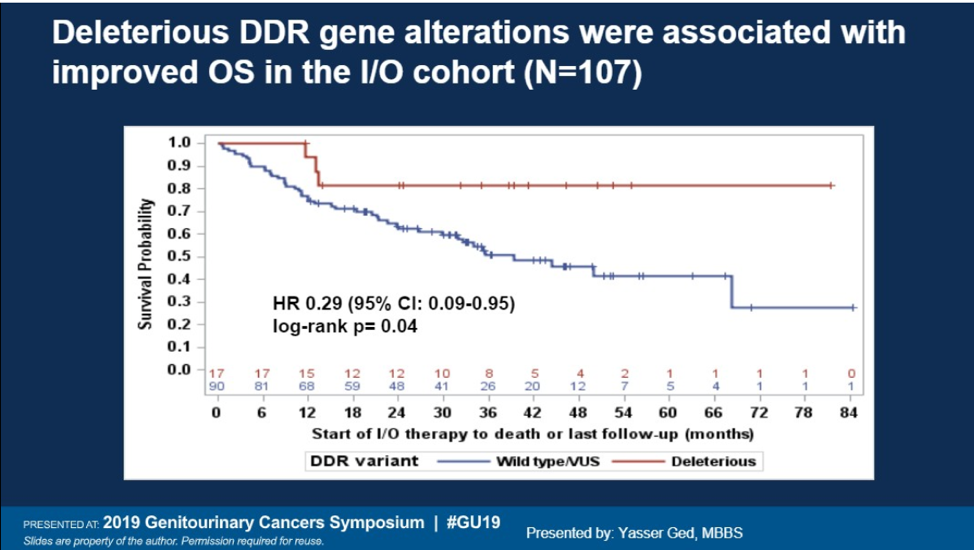
17% had a deleterious DDR gene alteration in this study. The most pathogenic variants were ATM, CHEK2, MSH6, and MUTY. 158 of the patients underwent germline testing and 7% were found to have pathogenic variants in DDR genes as well. Having a deleterious DDR gene alteration was associated with improved overall survival in the IO cohort (HR 0.29, p=0.04). No correlation with survival was seen with DDR mutations and OS in the TKI cohort. Additionally, deleterious DDR alterations were not associated with progression free survival in either cohort.
A significant percentage of patients with metastatic clear cell RCC have pathogenic variants in genes associated with DNA damage repair. These patients appear to have a survival advantage in patients without DDR alterations when treated with IO therapy and no advantage in TKI therapy. This was also seen in Abstract 589 (Labriola et al), which was also presented during this GU ASCO. Further prospective validation is necessary but these two studies suggest that DDR alterations may be prognostic for patients treated with IO.
Presented by: Yasser Ged, MBBS, MRCP, Memorial Sloan Kettering Cancer Center, New York, NY
Written by: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University, Twitter: @TheRealJasonZhu at the 2019 American Society of Clinical Oncology Genitourinary Cancers Symposium, (ASCO GU) #GU19, February 14-16, 2019 - San Francisco, CA
References:
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. New England Journal of Medicine 2015;373:1803-13.
- Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. New England Journal of Medicine 2018;378:1277-90.
- Zhu J, Armstrong AJ, Friedlander TW, et al. Biomarkers of immunotherapy in urothelial and renal cell carcinoma: PD-L1, tumor mutational burden, and beyond. Journal for ImmunoTherapy of Cancer 2018;6:4.
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. New England Journal of Medicine 2015;372:2509-20.
- Teo MY, Seier K, Ostrovnaya I, et al. Alterations in DNA Damage Response and Repair Genes as Potential Marker of Clinical Benefit From PD-1/PD-L1 Blockade in Advanced Urothelial Cancers. Journal of Clinical Oncology 2018;36:1685-94.


