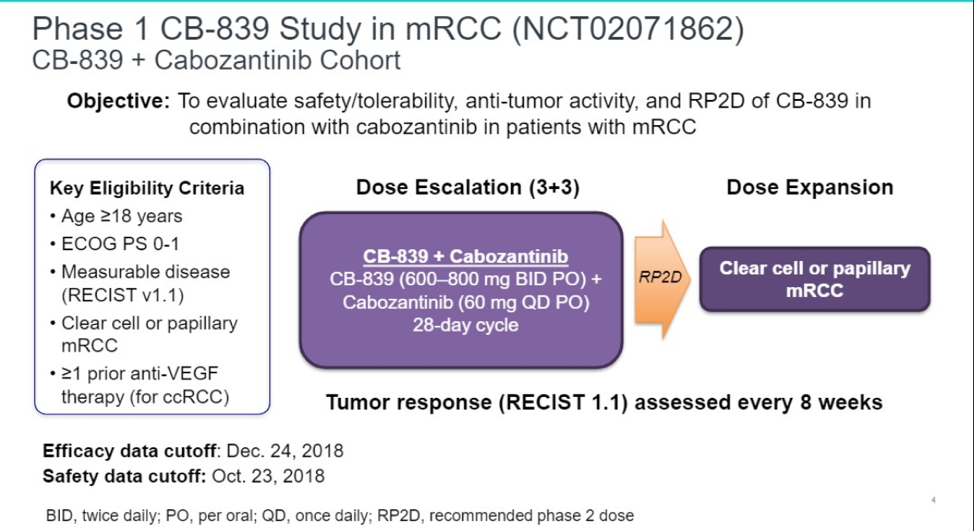
Summary: This is a phase I study evaluating CB-839, a first in clinic reversible oral glutaminase inhibitor. Inclusion criteria included patients with metastatic clear cell or papillary renal cell carcinoma with measurable disease, and for patients with clear cell disease, prior treatment with one or more anti-VEGF therapy. 800 mg twice a day was established as the recommended phase II dose with cabozantinib 60 mg daily. 13 patients have been enrolled.
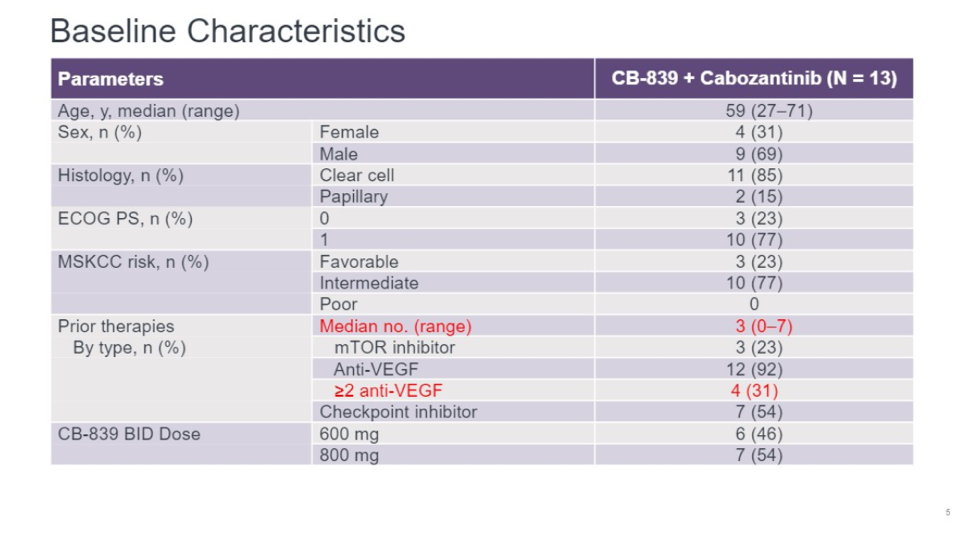
The median age was 59. This was a heavily pre-treated population with a median of 3 lines of prior therapies. 31% of patients had 2 or more anti-VEGF targeted agents. Half of the patients had been treated with an immune checkpoint inhibitor. There were no MSKCC poor risk patients.
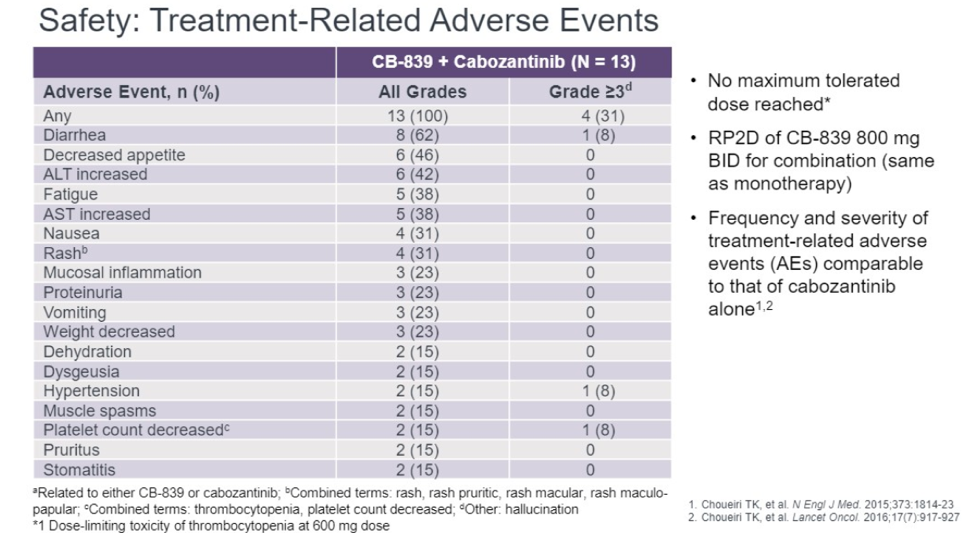
In terms of safety, the most common treatment related events were diarrhea (62%), decreased appetite (46%), elevated ALT (39%) or AST (31%), nausea (31%), and rash (31%). No maximum tolerated dose was reached and the recommended phase two dose is 800 mg BID.
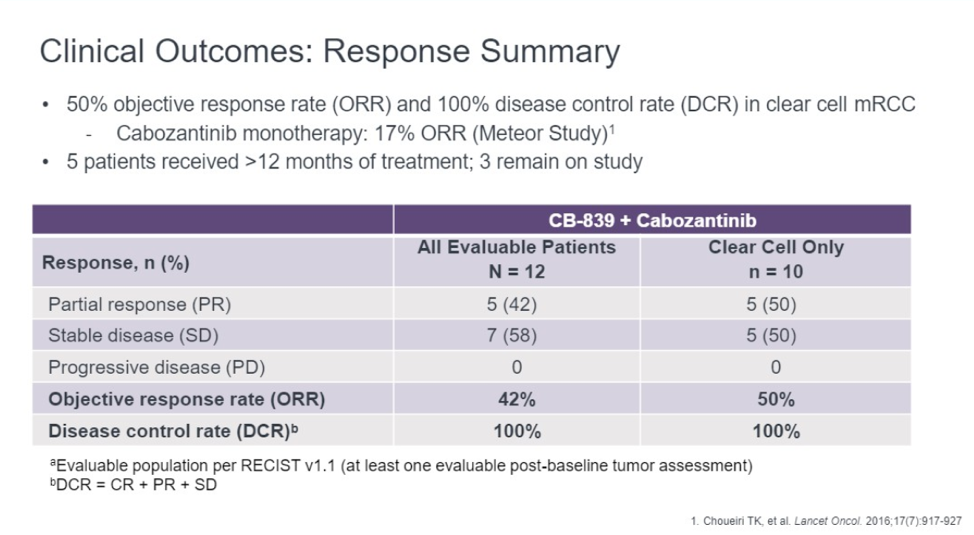
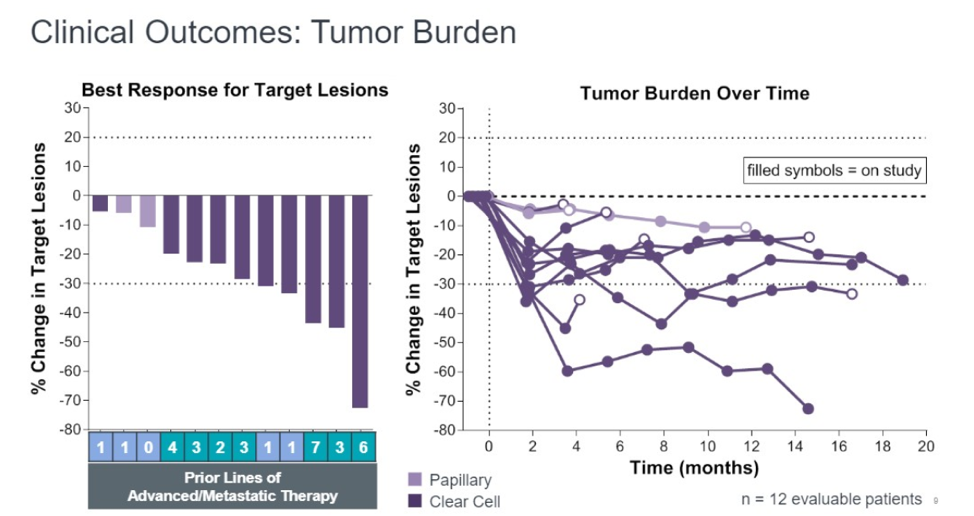
Among the 12 patients, 5 (42%) patients had an objective response. The remaining seven patients have stable disease. Of the original 12, 5 patients were treated for more than 12 months and 4 remain on therapy. Disease control rate, as defined as stable disease, complete response, or partial response was 100%.
Conclusions
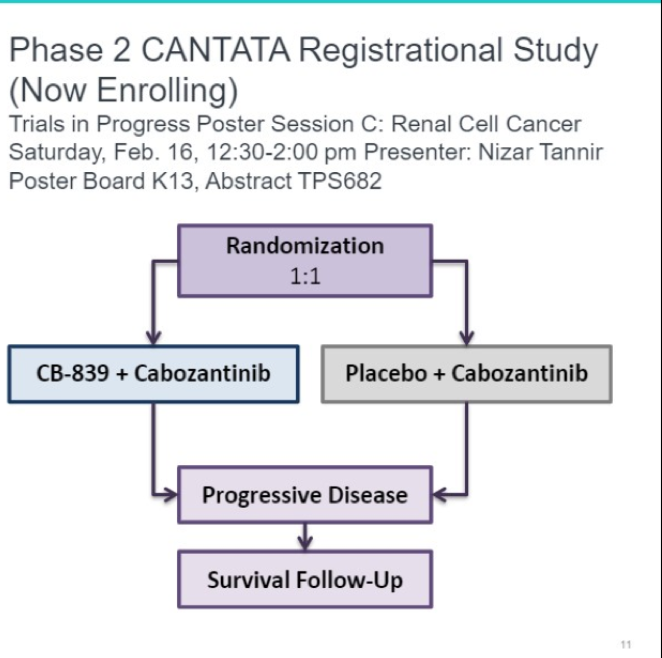
CB-839 is a novel small molecule which takes advantage of the glutamine metabolic pathway which is frequently upregulated in patients with renal cell carcinoma. These early results are exciting and a randomized phase II trial comparing CB-839 + cabozantinib to cabozantinib is underway (CANTATA).
Presented by: Funda Meric-Bernstam, MD, Department of Investigational Cancer Therapeutics, Division of Cancer Medicine, MD Anderson Cancer Center
Written by: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University, Twitter: @TheRealJasonZhu at the 2019 American Society of Clinical Oncology Genitourinary Cancers Symposium, (ASCO GU) #GU19, February 14-16, 2019 - San Francisco, CA
References:
- Medina MA, Sánchez-Jiménez F, Márquez J, Quesada AR, de Castro Núñez I. Relevance of glutamine metabolism to tumor cell growth. Molecular and cellular biochemistry 1992;113:1-15.
- Harding JJ, Telli ML, Munster PN, et al. Safety and tolerability of increasing doses of CB-839, a first-in-class, orally administered small molecule inhibitor of glutaminase, in solid tumors. Journal of Clinical Oncology 2015;33:2512-.


