There have been several trials assessing the efficacy of gemcitabine and oxaliplatin in advanced urothelial cell carcinoma as a better alternative. These have shown an objective response rate for this combination to range between 47-60%, and a median overall survival rate ranging between 6.5-15 months, as shown in table 1.
Table 1- Efficacy of gemcitabine + oxaliplatin in advanced urothelial carcinoma patients

In this rapid abstract presentation, Dr. Lee presented the COACH trial, which randomizing advanced urothelial carcinoma patients unfit for cisplatin-based chemotherapy to either Gemcitabine + carboplatin, or gemcitabine + oxaliplatin, Clinical trial information: NCT01487915 (Figure 1).
Figure 1 -Trial design:

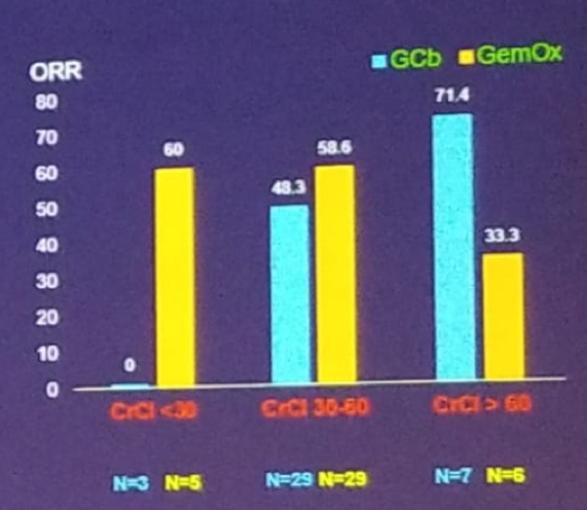
This study took place in eight tertiary academic centers between 2013 and 2017. The primary endpoint was response rate, and the secondary endpoints included progression-free survival, overall survival, and toxicity. The demographic and clinical data are shown in table 2. The overall response rate was shown to be 49% in the gemcitabine + carboplatin arm vs. 55% in the gemcitabine + oxaliplatin arm. The carboplatin arm seemed to be less active in patients with creatinine clearance of less than 30 ml/min while the oxaliplatin arm had preserved activity in these patients (figure 2).
Table 2 – Demographic and clinical data:
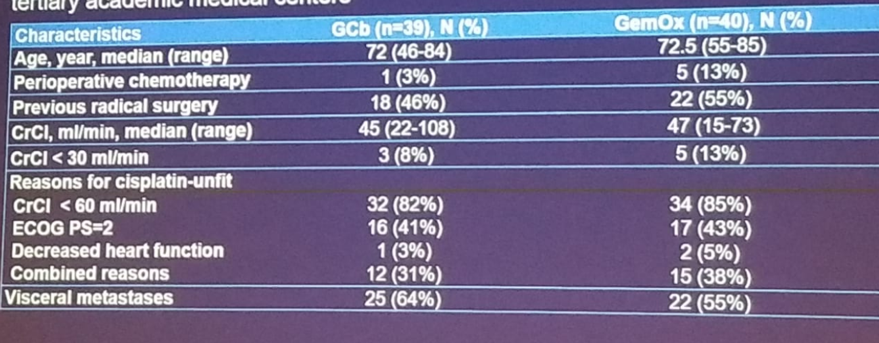
Figure 2 -Efficacy endpoints:
In conclusion, the gemcitabine + oxaliplatin regimen demonstrated comparable efficacy with the gemcitabine + carboplatin regimen, and favorable hematologic toxicity profile, at the expense of an increased rate of peripheral neuropathy. Dr. Lee concluded that Oxaliplatin may be used as a new option for patients who are not suitable for platinum-based chemotherapy.
Presented by: Jae-Lyun Lee, Asan Medical Center and University of Ulsan College of Medicine, Seoul, South Korea
Written By: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre @GoldbergHanan, at the 2019 American Society of Clinical Oncology Genitourinary Cancers Symposium, (ASCO GU) #GU19, February 14-16, 2019 - San Francisco, CA
References:
- De Santis et al.Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986.J Clin Oncol.2012 Jan 10;30(2):191-9. doi: 10.1200/JCO.2011.37.3571. Epub 2011 Dec 12.
- Poplin et al.Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: a trial of the Eastern Cooperative Oncology Group.J Clin Oncol. 2009 Aug 10;27(23):3778-85. doi: 10.1200/JCO.2008.20.9007. Epub 2009 Jul 6.


