Post-chemo retroperitoneal lymph node dissection (pcRPLND) is part of multimodal treatment for patients with NSGCT. It is currently indicated in patients with normalized or plateaued tumor markers with residual disease > 1 cm. The rationale to remove residual masses is based on the fact that in 40-45% and 10-15% of the cases, these patients harbor teratoma lesions or viable chemo-refractory germ cell tumors, respectively.
However, approximately 50% of patients are submitted to unnecessary surgery. This results from the fact that currently, there are no accurate tumor markers, imaging nor predictive models, enabling us to predict post-chemotherapy residual mass histology.
Fortunately, data involving micro RNA (miRNA) research could potentially help change the current standard paradigm. Recent published research identified a group of miRNAs with diagnostic properties in testicular germ cell tumors (TGCTs).1, 2 These miRNAs are specific and highly sensitive for TGCT, considerably more than the traditional tumor markers (Figure 1). These miRNA also demonstrated prognostic properties and seem to be correlated to disease burden, and potentially able to predict relapse.
Based on these results, the authors hypothesized that serum miRNA could serve as a predictive marker for viable disease in post-chemotherapy patients. An initial cohort of 39 patients whose serum was collected pre-chemotherapy, post-chemotherapy and after RPLND was analyzed. An additional cohort of 43 NGSCT (with the same clinical characteristics as the first cohort) whose serum was collected post-chemotherapy, was analyzed as well. All patients had NSGCT lesions, treated with orchiectomy, chemotherapy and pcRPLND. All the samples were evaluated for miR-371; 373 and 367 levels. The final cohort consisted of 14.6% of patients with viable GCT; 41.5% with teratoma and 43.9% harbored necrosis and fibrosis lesions.
In the study cohort the classical serum tumor markers (AFP, HCG and LDH) did not distinguish post-chemotherapy residual mass histology, not enabling to provide predictive information. However, miRNAs levels were associated with clinical stage with higher levels seen in patients with metastatic disease. A significant decrease in miR371 and miR367 levels during chemotherapy was seen. Interestingly, all miRNA levels significantly decreased from pre-chemo to post-RPLND period. All miRNA levels were higher in patients with viable TGCT. There were no differences between miRNA levels pre-chemo and post-chemo in patients with viable disease. There was a significant decline in patients with viable lesions after pcRPLND, but the same was not witnessed in patients with necrosis/fibrosis and teratoma lesions. The serum collected post-chemotherapy had significantly higher levels of miR-371 and miR-373 in patients with viable disease. Importantly, the miRNAs levels were similar between necrosis/fibrosis and teratoma lesions. Different combinations of the biomarkers were analyzed to predict viable TGCT post-chemotherapy. The most accurate combination was miR371+373; however, miR-371a-3p alone showed the highest discriminative capacity, with an [AUC of 0.874] figure 2.
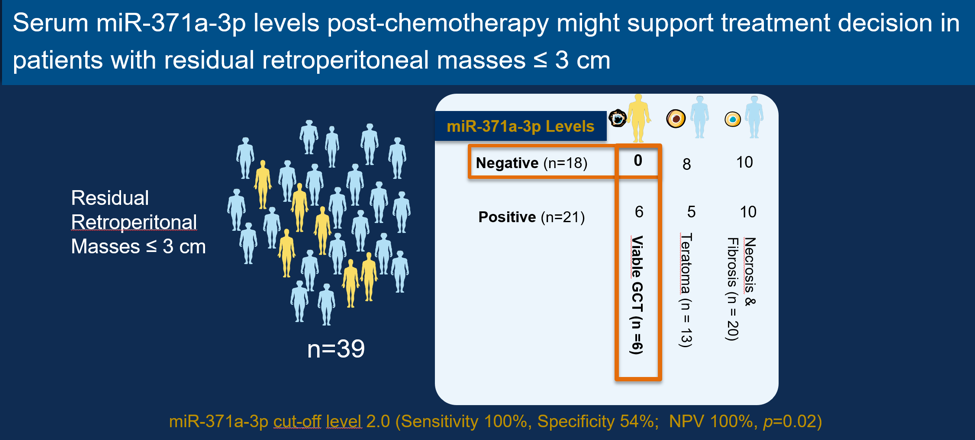
Finally, the authors assessed the clinical utility of their results. Given that the standard of care is to remove all residual masses >1cm, any avoidance of unnecessary pcRPLND would be desirable, provided it is safe. The authors examined whether small masses 1-3cm could potentially be observed if the miRNA test was negative. They observed that among patients with a negative miRNA, none had viable disease – reaching an impressive sensitivity of 100%, and a NPV of 100% (Figure 3).
Dr. Leao concluded his talk with the following key points. Serum miRNA are associated with clinical stage and treatment response. miR-371a-3p, as a single serum marker, accurately predicts viable disease post-chemotherapy. In a sub-group of patients with retroperitoneal lesions measuring ≤ 3 cm, miR-371a-3p levels might be able to support treatment decisions. Naturally, additional studies are required to validate these results.
Figure 1: Sensitivity of miRNA-371a-3p for testicular germ cell tumors:
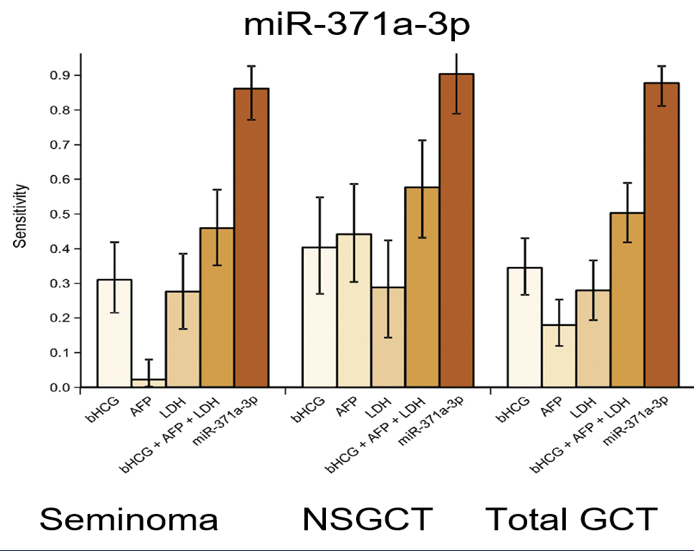
Figure 2: The ability of miRNA to predict the presence of viable GCTT post-chemotherpy:
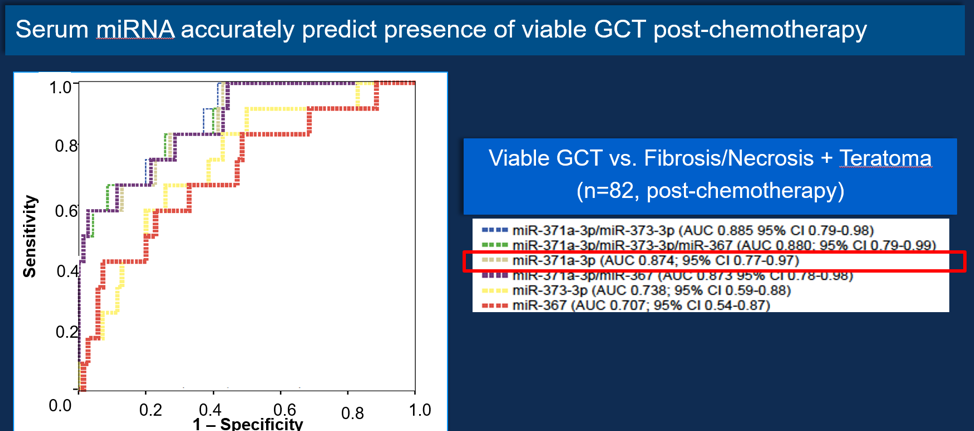
Figure 3 – Clinical utility of miRNA:
Presented by: Ricardo Romao Nazario Leao, MD, Princess Margaret Cancer Center, Toronto, Canada
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre @GoldbergHanan at the 2018 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, February 8-10, 2018 - San Francisco, CA
References:
1. van Agthoven T, Looijenga LHJ. Accurate primary germ cell cancer diagnosis using serum based microRNA detection (ampTSmiR test). Oncotarget 2017; 8(35): 58037-49.
2. Dieckmann KP, Radtke A, Spiekermann M, et al. Serum Levels of MicroRNA miR-371a-3p: A Sensitive and Specific New Biomarker for Germ Cell Tumours. European urology 2017; 71(2): 213-20.


