- Proof-of-concept study in first-line mRCC (a type of kidney cancer) shows that TECENTRIQ and Avastin can be combined with a manageable safety profile
- Study results also showed encouraging efficacy compared to sunitinib in those people whose disease expressed the PD-L1 (programmed death-ligand 1) protein
- Genentech is evaluating TECENTRIQ plus Avastin in a Phase III study (IMmotion151) in people with previously untreated, locally advanced or metastatic RCC
Orlando, Florida USA (UroToday.com) February 17, 2017 – Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), today announced encouraging results from the Phase II IMmotion150 study that compared TECENTRIQ® (atezolizumab) plus Avastin® (bevacizumab) and TECENTRIQ monotherapy to sunitinib alone in people with previously untreated, locally advanced or metastatic renal cell carcinoma (mRCC). These results were presented at the 2017 Genitourinary Cancers Symposium taking place from February 16-18 in Orlando, Fla. IMmotion150 is the first randomized clinical trial to evaluate the combination of TECENTRIQ and Avastin in mRCC. The study was designed to inform further clinical development of this combination, and these study results reinforce the potential of this combination in this setting.
The study showed that people whose disease expressed PD-L1 (programmed death-ligand 1) and were treated with TECENTRIQ plus Avastin had a 36 percent reduction in the risk of their disease worsening or death compared to people treated with sunitinib alone (median progression-free survival [mPFS]: 14.7 vs. 7.8 months; HR = 0.64; 95% CI 0.38, 1.08). No PFS advantage was observed in the overall study population (intention-to-treat [ITT]). Median duration of response (DoR) has not yet been reached after 20.7 months of follow-up across treatment arms. Adverse events in the TECENTRIQ plus Avastin arm were consistent with those observed in previous studies of each medicine.
“These Phase II results support the scientific rationale for potentially combining TECENTRIQ and Avastin in people with this type of kidney cancer,” said Sandra Horning, M.D., chief medical officer and head of Global Product Development. “There is a significant need for new treatment options for people living with advanced RCC, a disease where currently only about one in 10 people are alive beyond five years following diagnosis.”
Genentech is also evaluating TECENTRIQ plus Avastin compared to sunitinib in a Phase III study (IMmotion151; NCT02420821) in people with previously untreated, locally advanced or metastatic RCC. A study of TECENTRIQ as adjuvant treatment for RCC began enrolling earlier this year.
About the IMmotion150 study
IMmotion150 is a global, multicenter, open-label, randomized Phase II study that was designed to evaluate the efficacy and safety of TECENTRIQ plus Avastin (Arm A), TECENTRIQ alone (Arm B) or sunitinib alone (Arm C) in 305 people with previously untreated, locally advanced or metastatic RCC. People in Arm A received TECENTRIQ administered intravenously at 1200 milligrams (mg) every 3 weeks (6-week cycles) plus Avastin intravenously at 15 mg until disease progression or lack of clinical benefit. People in Arm B received TECENTRIQ alone (until disease progression or lack of clinical benefit), and people in Arm C received sunitinib 50 mg orally daily for 4 weeks followed by 2 weeks rest until disease progression.
The co-primary endpoints were PFS per RECIST v.1.1 via Independent Review Facility (IRF) assessment in all randomized patients (ITT population) and in the PD-L1- selected (IC1/2/3) subgroup. PD-L1 expression was assessed on tumor-infiltrating immune cells (IC) with an investigational immunohistochemistry (IHC) test based on the SP142 antibody being developed by Roche Tissue Diagnostics. Secondary endpoints included IRF-assessed overall response rate (ORR) and duration of response (DoR), investigator-assessed PFS, ORR, DoR and safety, and overall survival (OS). A summary of the efficacy data from Arms A, B and C of the IMmotion150 study is included below.
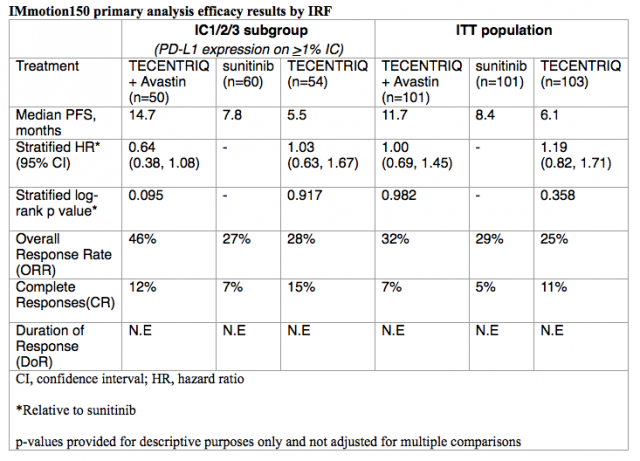
IMmotion150 was designed with planned crossover. Over three-quarters (78 percent) of people receiving sunitinib (Arm C) who progressed subsequently received TECENTRIQ plus Avastin (Arm A). OS results were immature at time of analysis with only 35 percent of events having occurred.
Safety in the TECENTRIQ plus Avastin arm appeared consistent with the known safety profile of the individual medicines. No new safety signals were identified. Frequency of all-grade treatment-related adverse events (AEs) was similar between arms. The most common AEs occurring in more than 20 percent of people receiving TECENTRIQ plus Avastin and with a greater than 5 percent increase when compared to sunitinib included: arthralgia (38 percent), proteinuria (36 percent), epistaxis (28 percent) and pruritus (22 percent). Frequency of grade 3-4 AEs regardless of relationship to treatment were similar between people treated with TECENTRIQ plus Avastin (63 percent) and sunitinib (69 percent). Treatment-related grade 3-4 events were reported in 40 percent of people treated with TECENTRIQ plus Avastin and in 57 percent of people treated with sunitinib. One person who was treated with TECENTRIQ plus Avastin experienced intracranial hemorrhage that led to death. Fifteen of 101 people (15 percent) treated with TECENTRIQ plus Avastin discontinued treatment for adverse events.
About renal cell carcinoma
Renal cell carcinoma (RCC) is the most common type of kidney cancer and forms when abnormal cells develop in the small tubes (known as renal tubules) in the kidneys. In 2017, about 64,000 people will be diagnosed with kidney cancer in the United States and more than 14,000 will die from the disease. The disease is more prevalent in men and people aged 55-74 years. Currently there is a significant need for more effective treatments with only about one in 10 people alive five years post-diagnosis.
About TECENTRIQ
TECENTRIQ is a monoclonal antibody designed to bind with a protein called PD-L1. TECENTRIQ is designed to bind to PD-L1 expressed on tumor cells and tumor-infiltrating immune cells, blocking its interactions with both PD-1 and B7.1 receptors. By inhibiting PD-L1, TECENTRIQ may enable the activation of T cells. TECENTRIQ may also affect normal cells.
About Avastin
Avastin is a prescription-only medicine that is a solution for intravenous infusion. It is a biologic antibody designed to specifically bind to a protein called vascular endothelial growth factor (VEGF) that plays an important role throughout the lifecycle of the tumor to develop and maintain blood vessels, a process known as angiogenesis. Avastin is designed to interfere with the tumor blood supply by directly binding to the VEGF protein to prevent interactions with receptors on blood vessel cells. The tumor blood supply is thought to be critical to a tumor's ability to grow and spread in the body (metastasize).
First Author: Ridwan Alam, BS, The Johns Hopkins University School of Medicine
at the 2017 Genitourinary Cancers Symposium - February 16 - 18, 2017 – Orlando, Florida USA


