(UroToday.com) The 2023 ASCO annual meeting included a testicular cancer session, featuring a presentation by Dr. Aditya Bagrodia discussing molecular drivers of organotropism and cisplatin resistance in germ cell tumors. Dr. Bagrodia started by highlighting that brain, bone, and liver metastases are associated with poor germ cell tumor outcomes:
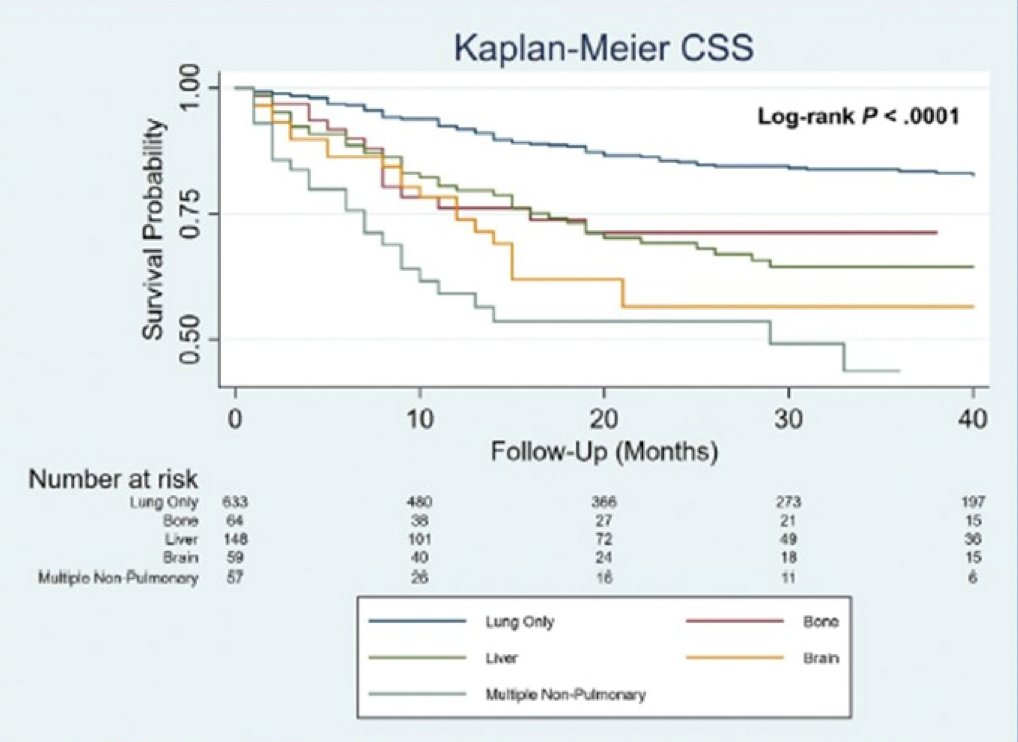
Cisplatin resistance occurs in up to 30% of patients with advanced germ cell tumors, and despite effective salvage treatment regimen, there is a 50% risk of cancer-specific death. Previously, Dr. Bagrodia’s group identified molecular features, including TP53 mutations and MDM2 amplification, associated with cisplatin resistance.1 Thus, perhaps there is an opportunity for novel options, including immunotherapy and targeted therapy. At ASCO 2023, Dr. Bagrodia discussed several objectives of their study:
- Identify molecular drivers of organotropism
- Identify molecular drivers of cisplatin-resistance
- Describe molecular differences in primary versus metastatic germ cell tumors
- Describe molecular differences in chemotherapy-naïve versus post-chemotherapy germ cell tumors
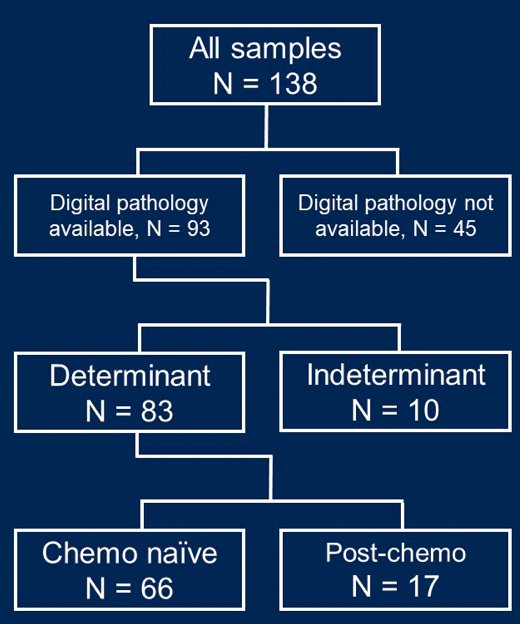
Germ cell tumors (n = 138) were tested at Caris Life Sciences with next-generation sequencing of DNA (592-gene or whole exome) and RNA (whole transcriptome). A specialized genitourinary pathologist blindly reviewed selected H&E-stained slides and designated tumors as chemotherapy naïve (n = 66) or post-chemotherapy (n = 17) based on treatment related changes:
Differentially regulated pathways were assessed by gene set enrichment analysis. Gene sets associated with platinum resistance were given a platinum sensitivity score, and gene values were added if associated with chemosensitivity and subtracted if associated with resistance. A high platinum sensitivity score is associated with more sensitivity to platinum resistance and a lower score is more resistant:

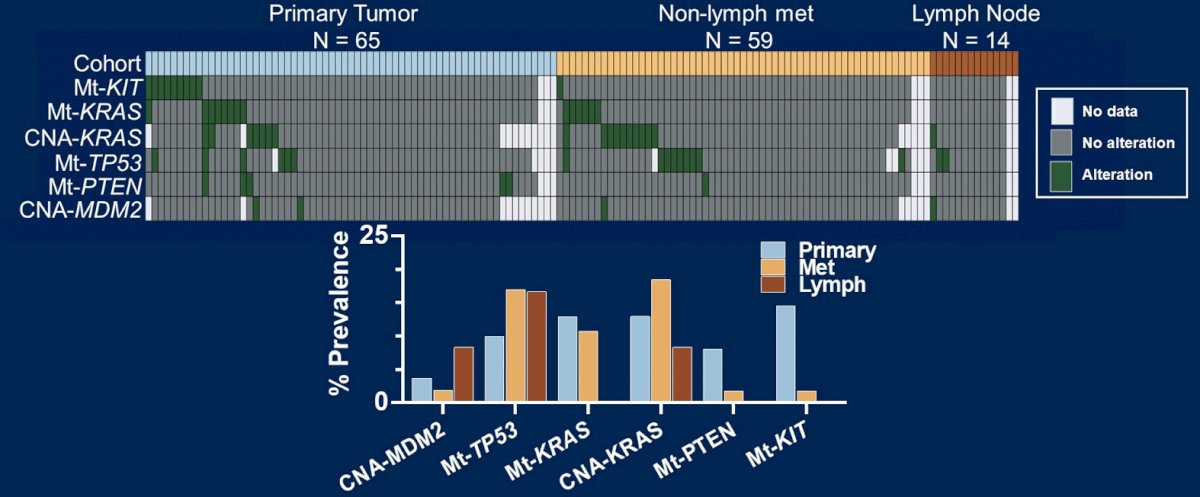
Primary (n = 65) and metastatic (n = 73) sites were defined based on the biopsy site. Mann-Whitney U and χ² tests were applied, with p-values adjusted for multiple comparisons.
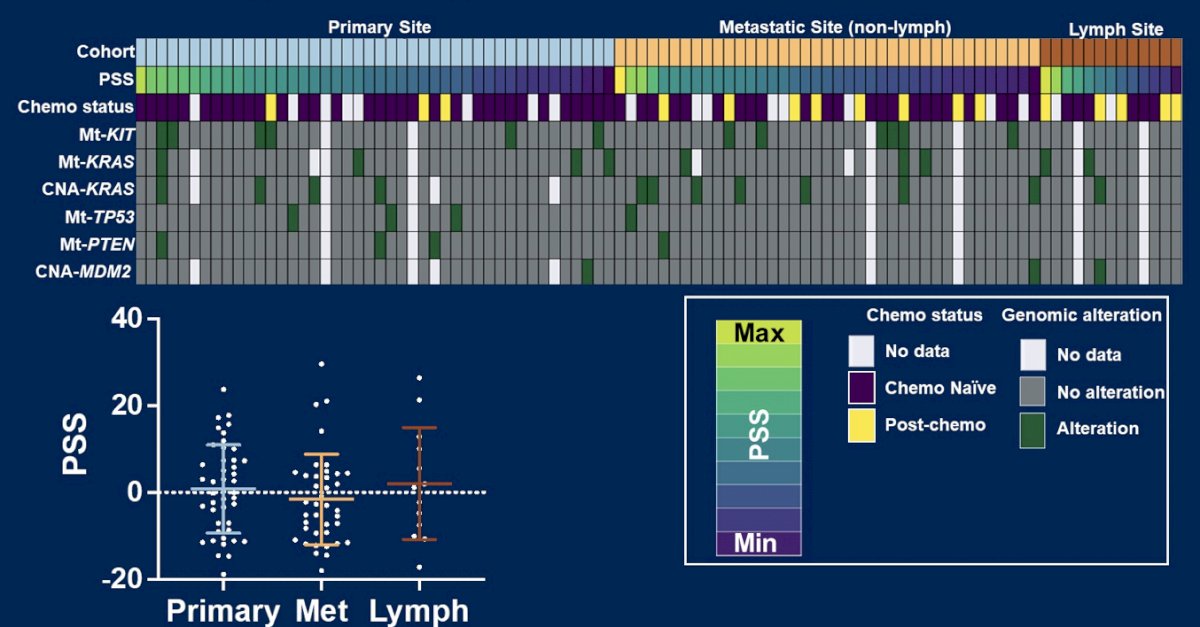
Primary and metastatic germ cell tumors had slight differences between putative drivers of platinum therapy resistance: KIT (15% vs 1%), TP53 (10% vs 17%), KRAS (13% vs 9%) mutations and MDM2 copy number amplifications (4% vs 3%), all p > 0.05:
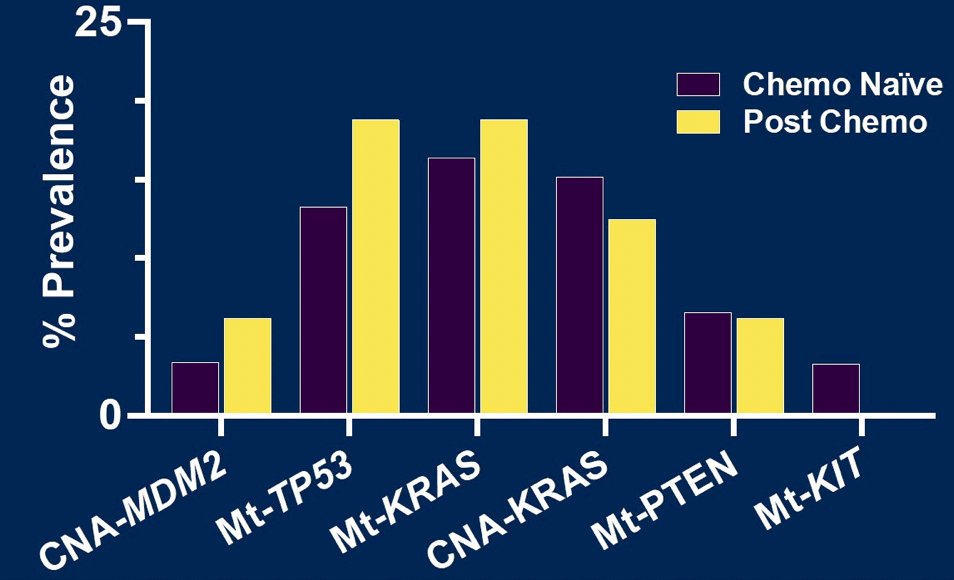
When comparing post-chemotherapy (n=17) vs chemotherapy naïve (n=66) germ cell tumors, there was no significant differences in the genomic landscape observed (p > 0.05):
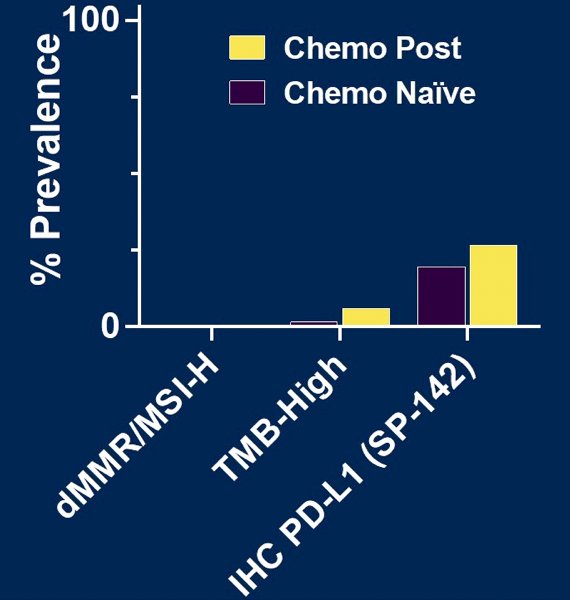
Furthermore, there was no significant difference between biomarker of response to immune checkpoint inhibitors, namely dMMR/MSI-H, TMB-high, and IHC PD-L1, for post-chemotherapy vs chemotherapy naïve germ cell tumors:
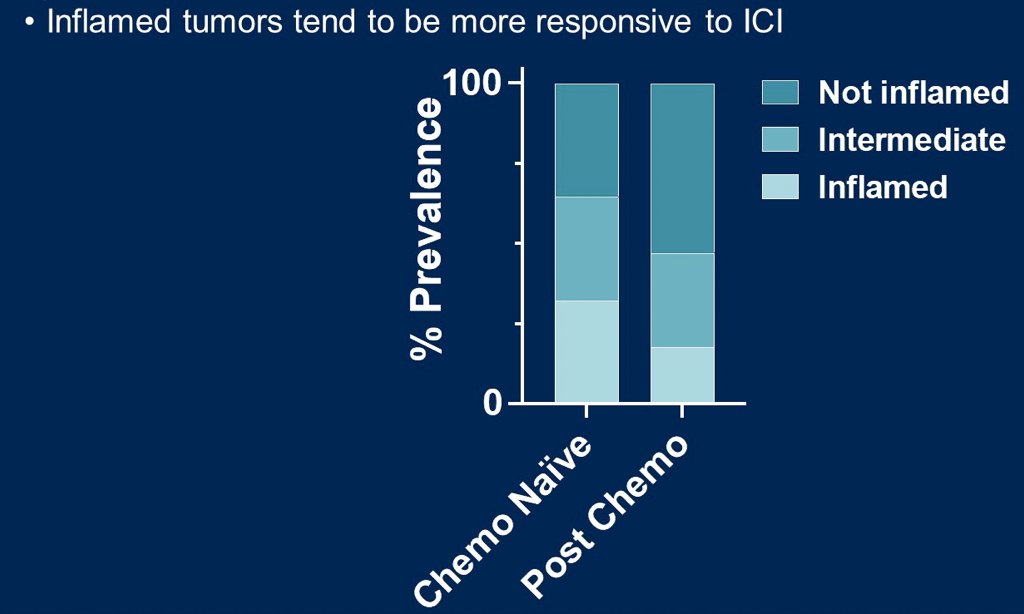
Chemo naïve tumors were shown to be enriched for gene sets associated with KRAS signaling and epithelial-to-mesenchymal transition. Additionally, they trended towards a higher proportion of T cell-inflamed tumors as compared to post-chemo samples (32% vs 18%, p = 0.36):
Interestingly, platinum sensitivity score was not significantly associated with tumor site:
Chemotherapy naïve tumors with TP53, KRAS, or KIT mutation or MDM2 copy number amplifications had a lower platinum sensitivity (-4.16 AU, n= 18) than tumors with no alterations (2.17, n = 38), p = 0.05. Finally, chemotherapy naïve tumors with MDM2 copy number amplifications (n = 2, -16.26 AU) had the lowest platinum sensitivity followed by tumors with mutations in TP53 (n = 6, -2.04), KIT (n = 2, -1.77) and KRAS (n = 9, -1.05).
Dr. Bagrodia concluded his presentation discussing molecular drivers of organotropism and cisplatin resistance in germ cell tumors with the following take-home points:
- There are no molecular alterations associated with site of metastasis: KIT mutations are more prevalent in primary tumors and TP53 mutations more prevalent in metastases
- No recurrent alterations were associated with platinum-resistance
- In chemotherapy-naïve tumors, significantly lower platinum sensitivity scores were associated with platinum-resistant alterations
- Limitations of this study include the small sample size and lack of clinical follow-up
Presented by: Aditya Bagrodia, MD, FACS, University of California San Diego Health, San Diego, CA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
References:
- Bagrodia A, Lee BH, Lee W, et al. Genetic determinants of cisplatin resistance in patients with advanced germ cell tumors. J Clin Oncol. 2016 Nov 20;34(33):4000-4007.


