(UroToday.com) The 2023 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between June 2nd and June 6th was host to a session on prioritizing and sequencing therapy for advanced prostate cancer. Dr. Julie Graff discussed the timing of Poly (adenosine diphosphate-ribose) polymerase inhibitors (PARP) inhibitors use for prostate cancer patients with defects in homologous recombination repair (HRR) genes.
Currently, there are two PARP inhibitors that are FDA approved for the treatment of prostate cancer patients: olaparib and rucaparib. These drugs are approved in the metastatic castrate-resistant prostate cancer (mCRPC) setting either before or after chemotherapy, as monotherapy drugs. While rucaparib is only FDA approved for patients with BRCA 1/2 mutations, olaparib is approved for those with any of the mutations eligible for either Cohort A (BRCA1, BRCA2, ATM) or Cohort B of the PROfound trial, despite the majority of the survival benefit for olaparib in this trial being derived from those with BRCA1/2 mutations.1 As of May 31st, 2023, the FDA approved abiraterone/prednisone + olaparib in mCRPC patients with BRCA1 or BRCA2 mutations, based on results of PROpel.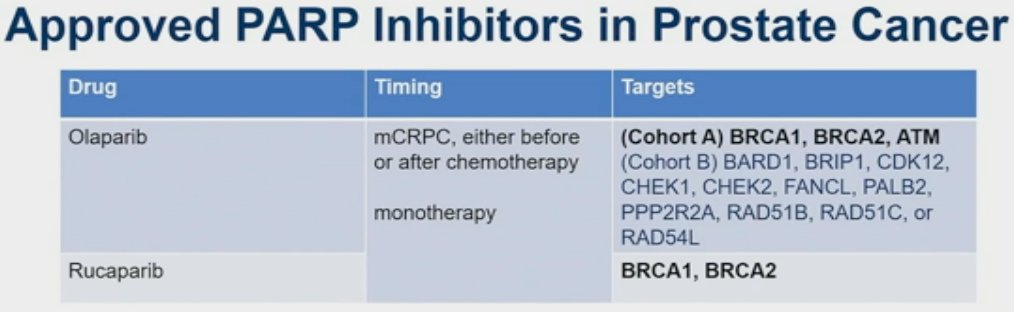
While it is clear that PARP inhibitors work well in those with HRR mutations, is there a role for these drugs in patients without an HRR deficiency? And in addition to the mCRPC disease state, are there other disease stages for which PARP inhibitors should be used for those with an HRR deficiency?
PARPs are involved in the repair of single strand DNA breaks. PARP inhibitors prevent this single strand repair mechanism and induce the creation of double strand breaks. However, it is only in those HRR deficient cells that these double strand breaks result in subsequent cell death. This is reinforced by PARP inhibitors demonstrating efficacy only for select mutations, as summarized below. These HRR mutations can be inherited (germline), acquired (somatic), or potentially induced, as has been the focus of recent studies.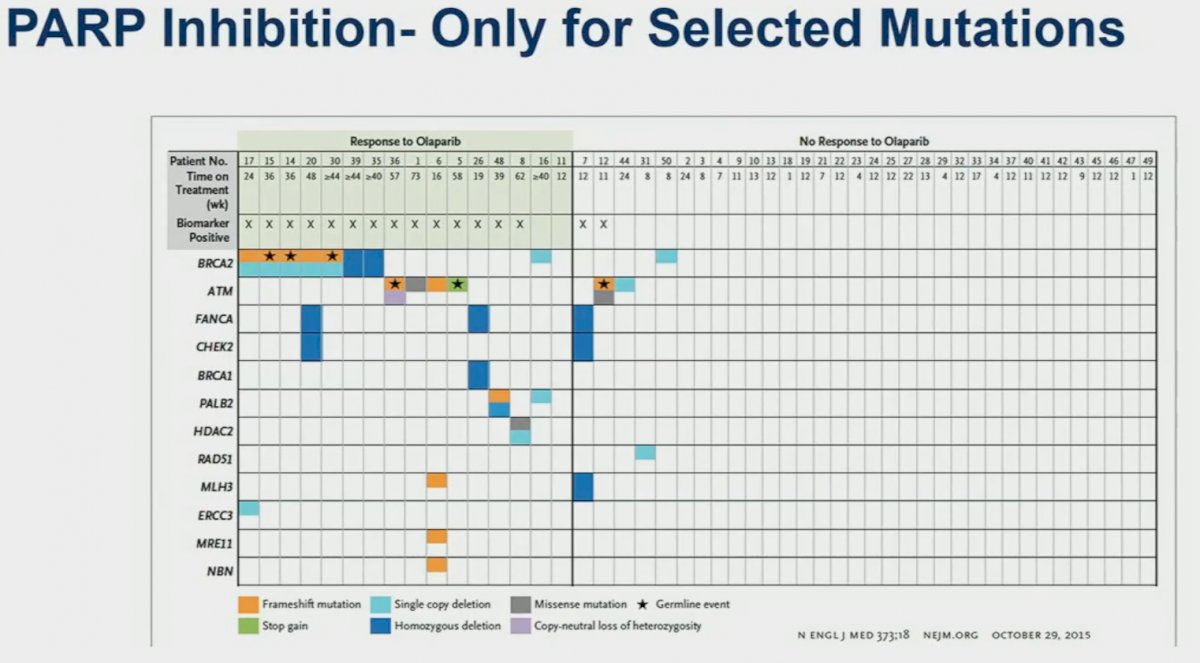
Preclinical models have suggested synergistic mechanisms of action for PARPi and androgen receptor signaling inhibitors (ARSIs). PARPi upregulates androgen receptor signaling, enhancing ARSI activity. Conversely, ARSIs inhibit the transcription of some HRR genes, inducing an HRR deficiency-like state, thus potentiating PARPi activity.3-5 These mechanisms of action suggest that the PARPi/ARSI combination may have clinical efficacy, irrespective of HRR mutational status. This was further supported by the results of a phase II trial of combination olaparib + abiraterone acetate that demonstrated improved radiographic progression-free survival (rPFS) in docetaxel pre-treated, HRR biomarker unselected mCRPC patients.6 This is illustrated in the lower left panel, that demonstrates evidence of a marginal rPFS benefit for this combination in the HRR negative cohort (HR: 0.52, 95% CI: 0.24 – 1.15, p=0.11).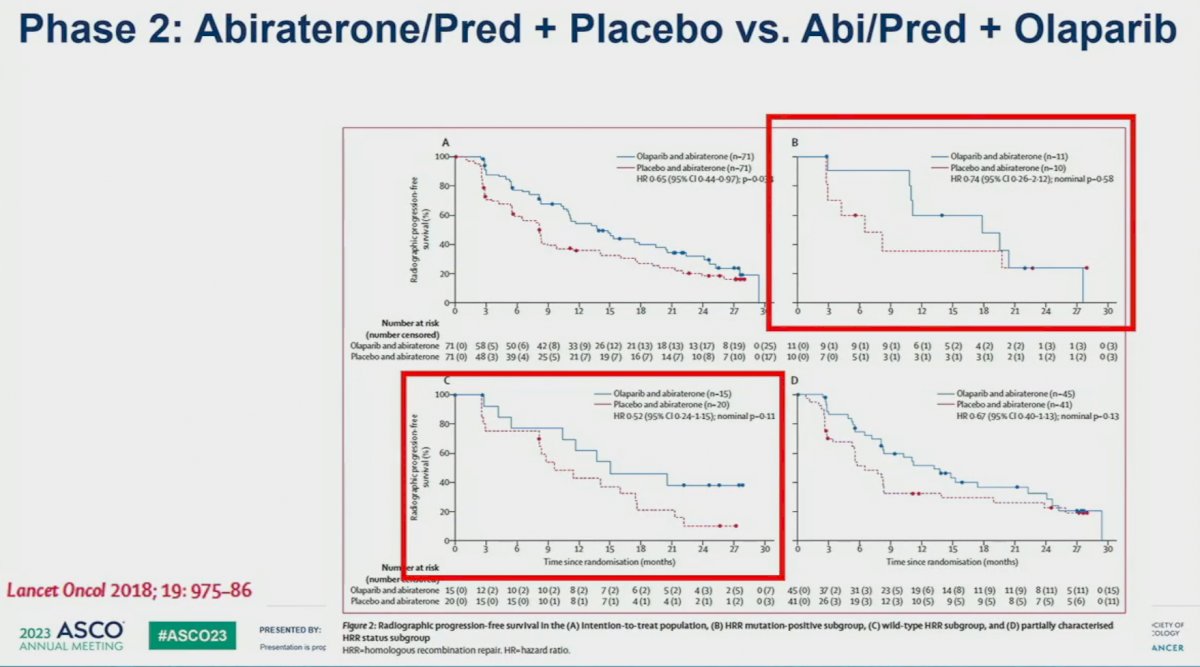
Over the last year, three phase III trials evaluating the combination of an ARSI + a PARP inhibitor in the 1st line treatment setting for mCRPC patients have been published:
- MAGNITUDE: Abiraterone acetate/prednisone +/- niraparib7
- PROpel: Abiraterone acetate/prednisone +/- olaparib2
- TALAPRO: Enzalutamide +/- talazoparib8
MAGNITUDE is a phase III, randomized, double-blind, placebo-controlled, multicenter trial that evaluated the combination of niraparib plus abiraterone acetate/prednisone in mCRPC patients receiving first-line treatment. In contrast to PROpel and TALAPRO-2, MAGNITUDE included biomarker pre-screened cohorts, meaning that all patients had HRR mutational status determined prior to study enrollment. HRR mutational status was determined using tissue and/or blood samples. Patients with a gene alteration (ATM, BRCA1/2,, BRIP1, CDK12, CHEK2, FANCA, HDAC2, or PALB2) detected by ≥1 assay were assigned to the HRR+ cohort, whereas those with both assays negative were included in the HRR- cohort. Patients in the HRR+ and HRR- cohorts underwent 1:1 randomization to receive either reduced-dose niraparib 200 mg once daily (usual dose: 400 mg) and abiraterone acetate 1,000 mg once daily plus prednisone 5 mg twice daily or placebo + abiraterone/prednisone. Given the lower likelihood of clinical benefit with combination treatment in the HRR- cohort, a futility analysis was preplanned when approximately 200 patients had been enrolled and approximately 125 composite end point events (the first of either PSA progression, radiographic progression, or death) had occurred. For the HRR+ cohort, primary and secondary endpoints were tested using a pre-specified strategy. The primary rPFS endpoint was powered for and tested first in the BRCA1/2 subgroup and if statistical significance was reached, the remainder of the HRR+ patients would be analyzed. Approximately 50% of HRR+ patients were specified to be BRCA1/2 positive.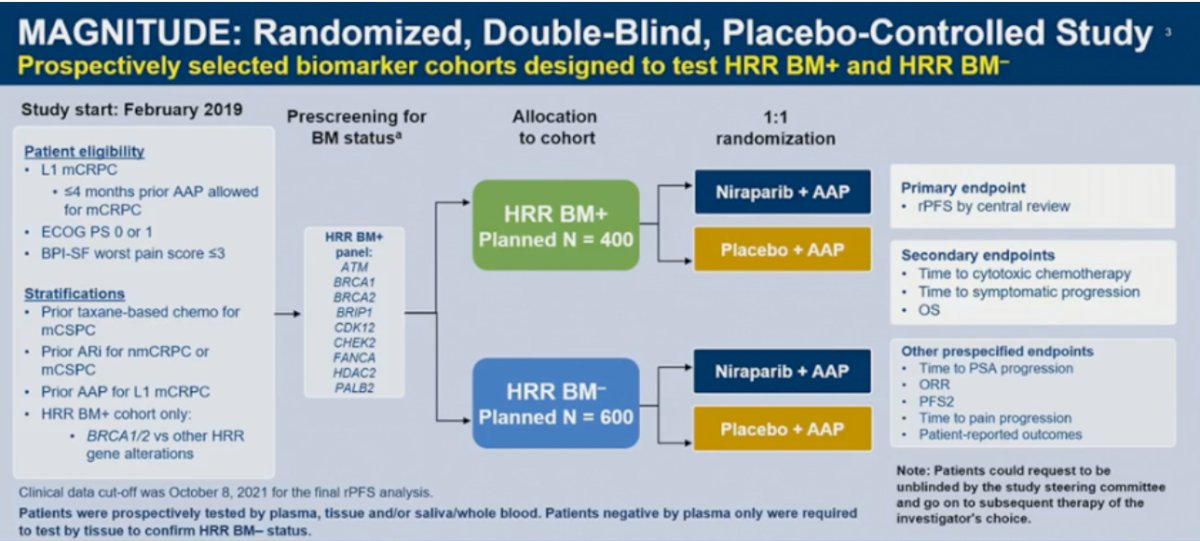
Results of the futility analysis in the HRR- cohort (n=233) demonstrated no benefit for combination niraparib/abiraterone versus placebo/abiraterone. The hazard ratio for the composite endpoint was 1.09, with futility defined as a hazard ratio greater than 1.0. As anticipated, additional grade ≥3 AEs occurred with addition of niraparib. Given the increased toxicity with no observed oncologic efficacy, the independent data monitoring committee recommended suspending enrollment in the HRR- cohort.
In the BRCA1/2 subgroup, median rPFS, assessed via blinded independent central review (BICR), was significantly prolonged with combination niraparib/abiraterone (16.6 versus 10.9 months; HR: 0.53, 95% CI: 0.36 – 0.79, p=0.001). A similar rPFS benefit with combination niraparib/abiraterone was next observed in the HRR+ cohort (16.5 versus 13.7 months; HR: 0.73, 95% CI: 0.56 – 0.96, p=0.022). OS data remain immature, with only 46.3% of the required events for final analysis having occurred. Crude, unadjusted analysis demonstrated an OS benefit of only 6% in the HRR+ cohort (HR: 0.94, 95% CI: 0.65 – 1.36). However, pre-specified, multivariate analysis OS, adjusting for baseline variables, favored the niraparib/abiraterone arm (HR: 0.77, 95% CI: 0.53 – 1.12).
PROpel is a global, randomized, double-blind phase 3 trial of abiraterone and olaparib versus abiraterone and placebo in patients with mCRPC treated in the first-line setting. Patients in PROpel were enrolled irrespective of HRRm status, ascertained via circulating tumor DNA (ctDNA) or tissue testing. Patients were randomized (1:1) to receive abiraterone (1000 mg once daily) plus prednisone/prednisolone with either full dose olaparib (300 mg twice daily) or placebo.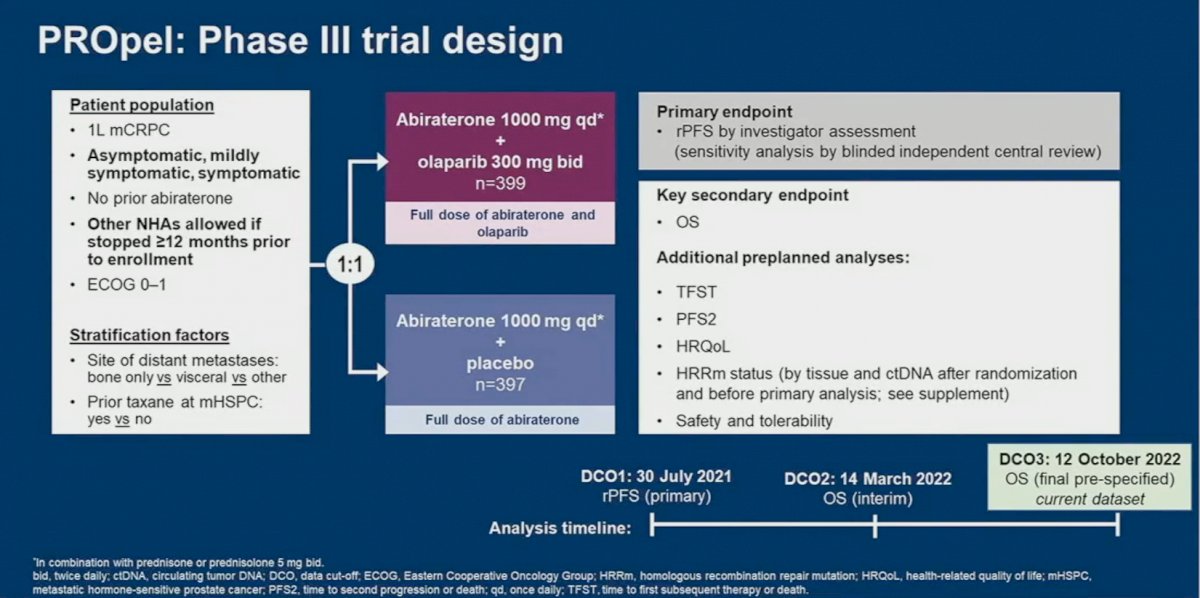
At a median follow-up of 19.4 months, the combination of olaparib/abiraterone significantly improved median investigator-assessed rPFS from 16.6 to 24.8 months (HR: 0.66, 95% CI: 0.54 – 0.81, p<0.001). Consistent results were observed when rPFS was assessed via blinded-independent central review (BICR).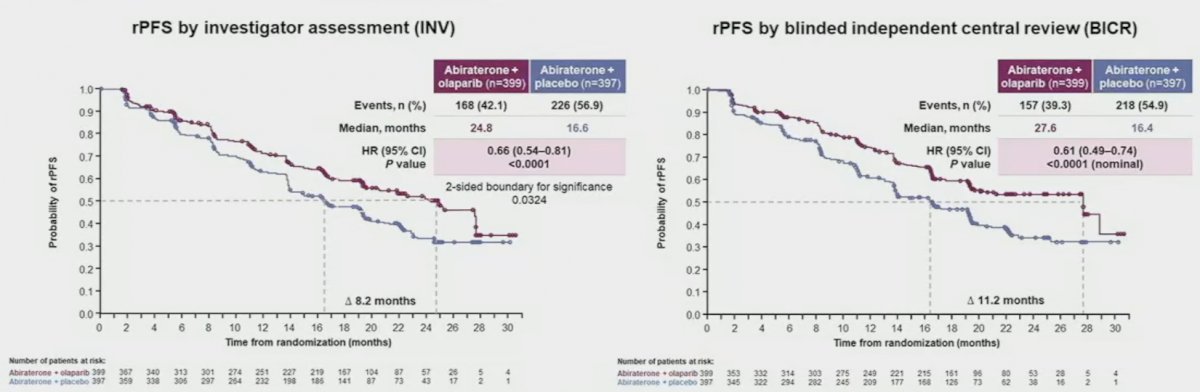
rPFS benefits were observed irrespective of HRRm status, although, as expected, the magnitude of effect was higher in the HRRm (HR: 0.50, 95% CI: 0.34 – 0.73) versus non-HRRm patients (HR: 0.76, 95% CO: 0.60 – 0.97). On subgroup analysis, rPFS benefits were observed irrespective of prior docetaxel exposure and metastatic site. The pre-planned final OS analysis was presented at ASCO GU 2023, with a consistent trend towards an OS benefit with combination olaparib/abiraterone in the overall intention-to-treat (ITT) population (data maturity: 47.9%; HR 0.81, 95% CI: 0.67 – 1.00, p=0.054), with median OS of 42.1 and 34.7 months, respectively.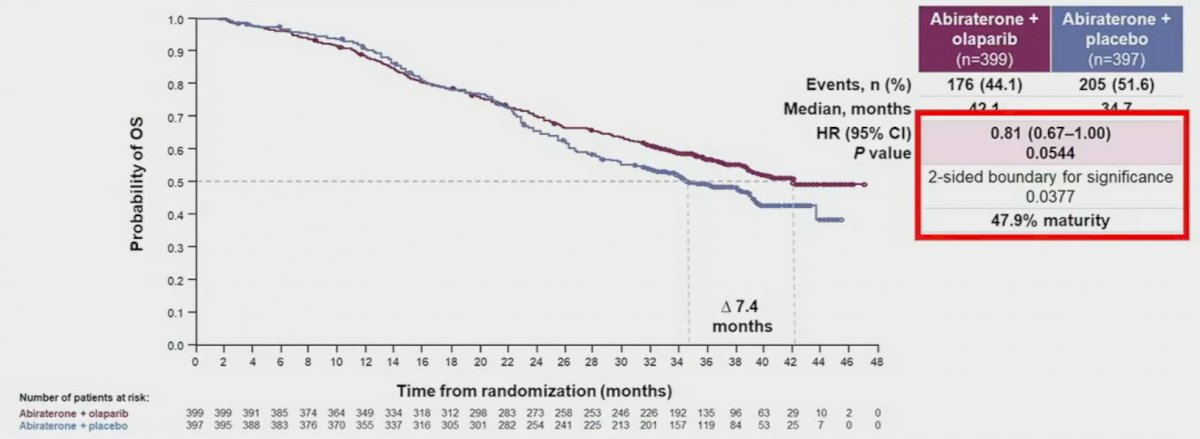
When OS is stratified by HRR mutational status, we observe a significantly larger magnitude of benefit in the HRRm cohort (median OS: not reached versus 28.5 months; HR: 0.66, 95% CI: 0.45 – 0.95), compared to those without HRR mutations (median OS: 42.1 months versus 38.9 months; HR: 0.89, 95% CI: 0.70 – 1.14).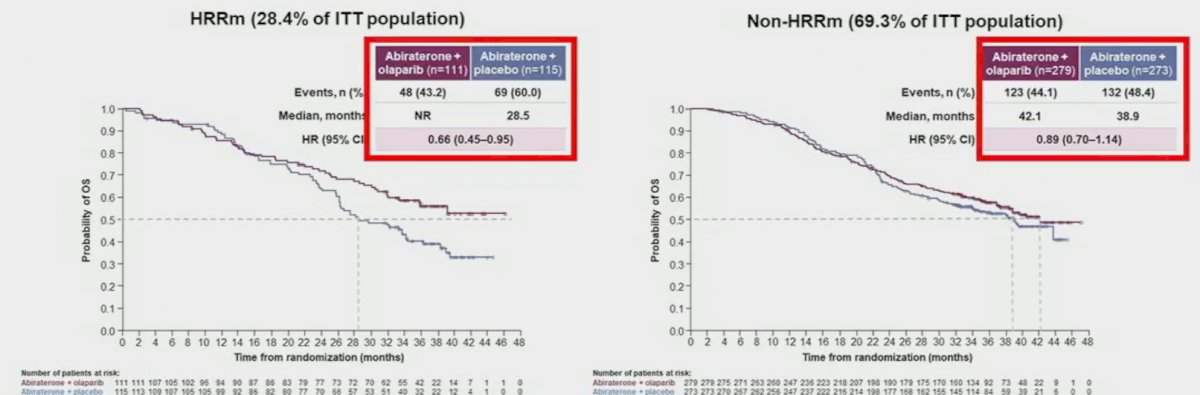
TALAPRO-2 is a phase III randomized, double-blind, placebo-controlled trial that evaluated the combination of talazoparib and enzalutamide in the 1st line treatment setting for mCRPC patients. Patients were randomized 1:1 to talazoparib 0.5 mg once daily (reduced dose from standard of 1.0 mg) plus enzalutamide 160 mg once daily versus placebo + enzalutamide.
Similar to PROpel, this was a biomarker unselected cohort of ‘all comers’, although randomization was stratified by HRR gene alteration status (deficient versus non-deficient or unknown).
The combination of talazoparib/enzalutamide was associated with a 37% decrease in the rPFS hazard, compared to placebo/enzalutamide (median: not reached versus 22 months; HR: 0.63, 95% CI: 0.51 – 0.78, p<0.001). 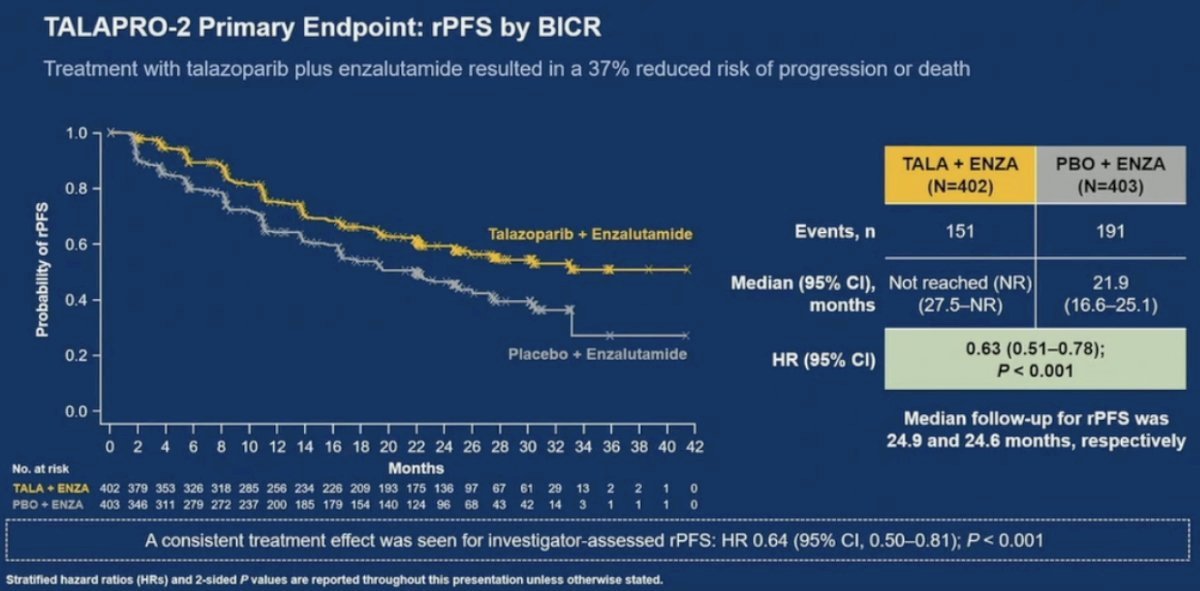
Similar to PROpel, rPFS benefits were observed in both the HRR mutated and non-mutated subgroups. As expected, the magnitude of effect was larger in the HRR mutated (median rPFS: 28 versus 16 months; HR: 0.46, 95% CI: 0.30 – 0.70, p<0.001) versus non-mutated subgroup (HR: 0.66, 95% CI: 0.49 – 0.91, p=0.009). OS data remains immature, with a trend towards an OS improvement in the overall cohort (HR: 0.89, 95% CI: 0.69 – 1.14, p=0.35).
Dr. Graff concluded her presentation with the following take-home messages:
- Every patient with metastatic disease should have genetic testing for germline and somatic mutations
- mCRPC patients with HRR mutations should be considered for PARP inhibitor monotherapy following progression on enzalutamide/abiraterone
- Patients with progressive disease not harboring HRR mutations should be considered for chemotherapy or an alternate to PARP inhibitor therapy
Presented by: Julie N. Graff, MD, Professor, OHSU Knight Cancer Institute, Portland, OR
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
References:- de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med, 2020;382(22):2091-2102.
- Clarke N, Armstrong AJ, Thiery-Vuillemin A, et al. Abiraterone and olaparib for metastatic castration-resistant prostate cancer. NEJM Evidence, 2022.EVIDoa2200043.
- Asim M, Tarish F, Zecchini HI, et al. Synthetic lethality between androgen receptor signalling and the PARP pathway in prostate cancer. Nat Commun, 2017; 8: 374.
- Schiewer MJ, Goodwin JF, Han S, et al. Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov, 2012; 2: 1134-49.
- Li L, Karanika S, Yang G, et al. Androgen receptor inhibitor-induced “BRCAness” and PARP inhibition are synthetically lethal for castration-resistant prostate cancer. Sci Signal, 2017; 10: eaam7479.
- Clarke N, Wiechno P, Alekseev B, et al. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol, 2018; 19: 975-86.
- Chi KN, Rathkopf D, Smith MR, et al. Niraparib and Abiraterone Acetate for Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol, 2023;JCO2201649.
- Agarwal N, Azad A, Carles J, et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): a randomised, placebo-controlled, phase 3 trial. The Lancet, 2023.


