(UroToday.com) The 2023 ASCO annual meeting included a prostate cancer session, featuring a presentation by Dr. Daniel Song discussing the REASSURE study assessing real-world safety and effectiveness of Radium-223 in patients with metastatic castration-resistant prostate cancer (mCRPC) treated in the US. Radium-223 improved overall survival and quality of life and demonstrated a favorable safety profile in patients with mCRPC in the phase 3 ALSYMPCA trial.1 REASSURE (NCT02141438) is a global, prospective, single-arm, observational study of Radium-223 use in patients with mCRPC and bone metastases in routine clinical practice with 7 years of follow-up. The REASSURE primary interim analysis confirmed that the short-term safety profile of Radium-223 was comparable to that seen in ALYMPCA, regardless of prior chemotherapy use. At the ASCO 2023 annual meeting, Dr. Song and colleagues presented clinical outcomes from the second planned interim analysis of REASSURE for patients treated in the US.
This analysis included patients with confirmed mCRPC with bone metastases scheduled to receive Radium-223 in the US, including all patients who received ≥1 dose of Radium-223. The study design for REASSURE is as follows:
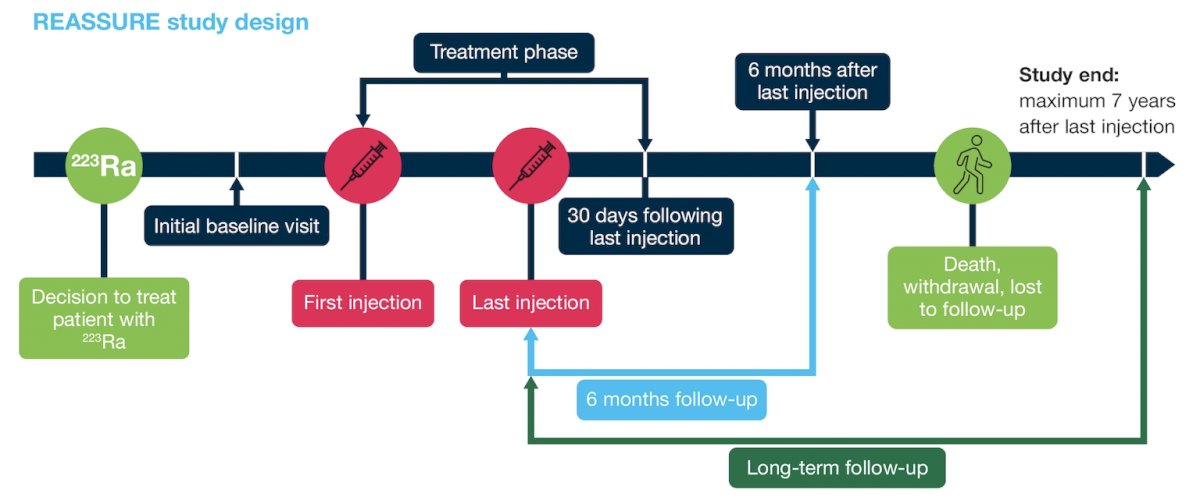
The primary endpoints are short- and long-term safety, including incidence of bone marrow suppression and second primary malignancies. Secondary endpoints included overall survival and patient-reported pain (Brief Pain Inventory – Short Form [BPI–SF] scores). A clinically meaningful pain response was defined as a decrease from baseline of ≥2 points in BPI-SF worst pain item.
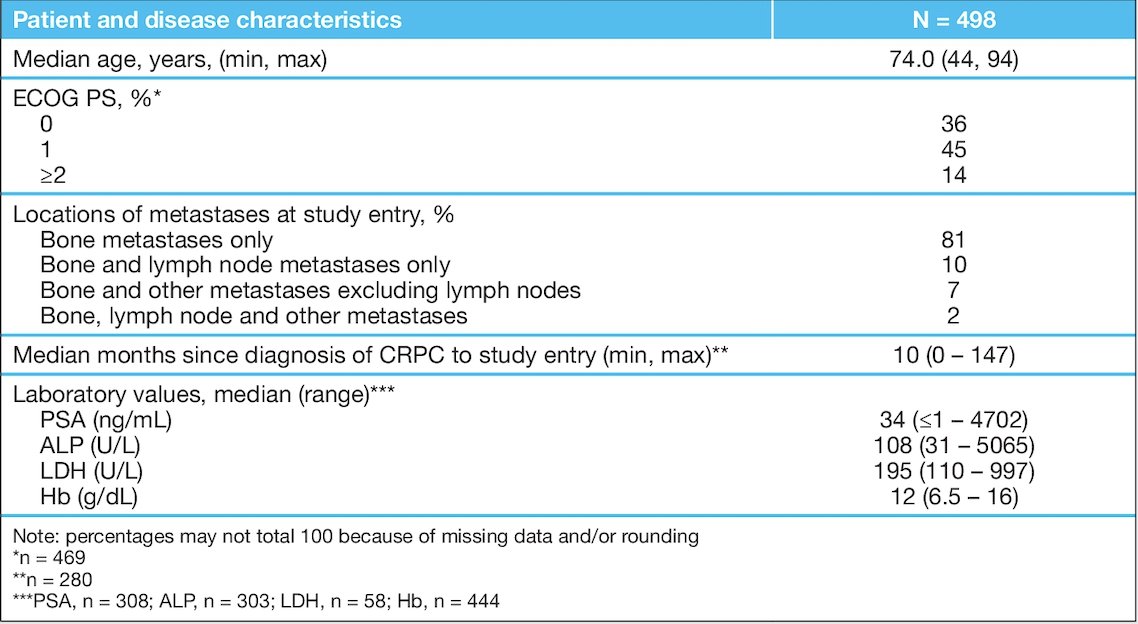
Patients in REASSURE were enrolled from 2014–2017, with 498 patients included in this analysis. At the data cut-off (March 20, 2019), the median duration of observation was 11.9 months (range: 0.4–41.3). Most patients (81%) had bone metastases only, and 69% of patients received 5–6 Radium-223 injections. The complete baseline characteristics are as follows:
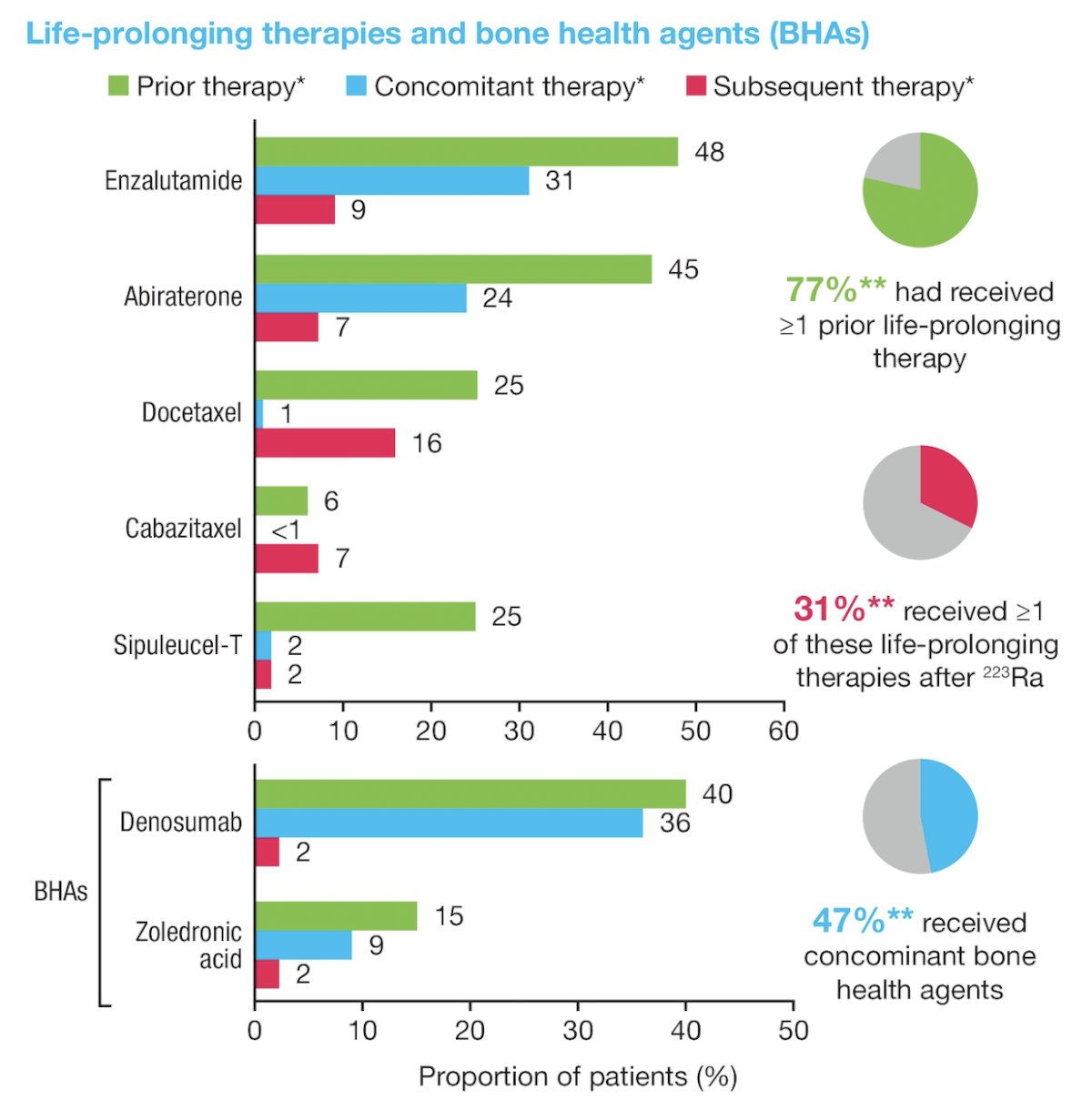
Overall, 77% of patients had received ≥1 prior life-prolonging therapies, which included abiraterone (45%), enzalutamide (48%), docetaxel (25%), cabazitaxel (6%), or sipuleucel-T (24%). Of specific interest, concomitant enzalutamide was received by 31% of patients, and 47% received concomitant bone health agents. After Radium-223, 31% of patients received ≥1 life prolonging therapies. A summary of life-prolonging therapies and bone health agents is as follows:
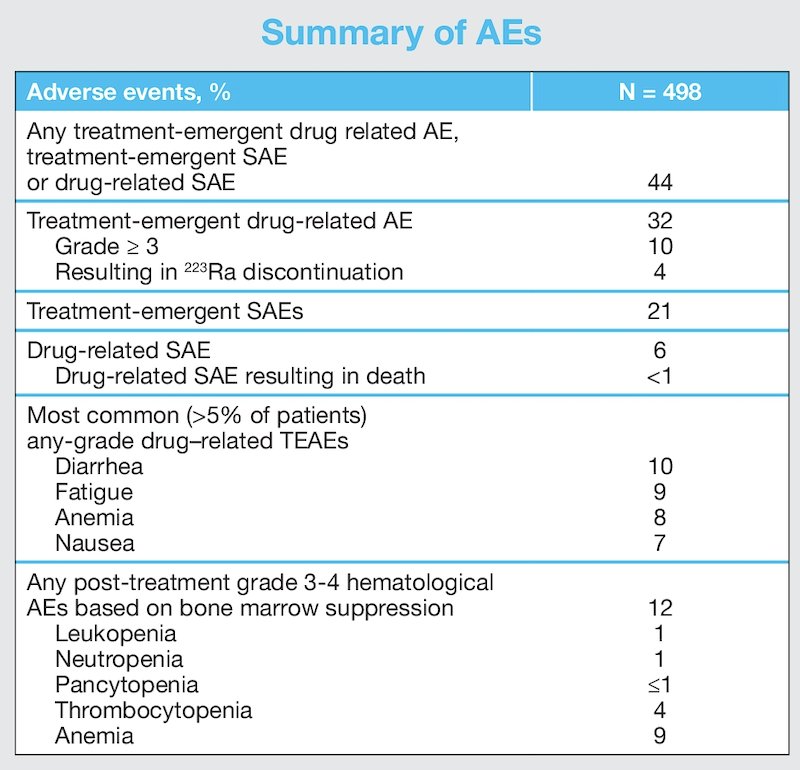
During treatment, 208/358 (58%) patients with a baseline BPI-SF ≥2 had a clinically meaningful pain response. Any-grade and grade ≥3 drug-related treatment-emergent adverse events occurred in 32% and 10% of patients, respectively. Drug-related treatment-emergent adverse events resulted in Radium-223 discontinuation in 4% of patients. Treatment-emergent and drug-related serious adverse events occurred in 21% and 6% of patients, respectively. The most common (>5% of patients) any-grade drug-related treatment-emergent adverse events were diarrhea (10%), fatigue (9%), anemia (8%) and nausea (7%).
Overall, 4% of patients had fractures and 2% of patients developed bone disorders. There were 11 second primary malignancies occurring in 10 patients (2%). In total, 60% of patients died during study follow-up, with a median overall survival of 17.8 months (95% CI 15.6–19.4):
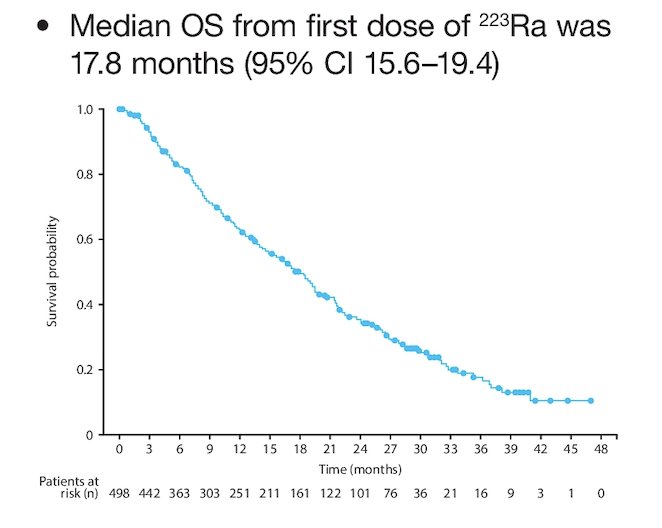
Dr. Song concluded his presentation discussing the REASSURE study assessing real-world safety and effectiveness of Radium-223 in patients with mCRPC treated in the US by highlighting the following take-home points:
- Within the current treatment landscape in routine clinical practice in the US, median overall survival after Radium-223 treatment was close to 18 months
- A majority of patients completed 5–6 Radium-223 injections
- The known safety profile of Radium-223 was confirmed with no new safety signals
- Over half of patients with pain at baseline had a clinically meaningful pain response during Radium-223 treatment
- Treatment with Radium-223 did not preclude patients from receiving subsequent therapies, including chemotherapy
Presented by: Daniel Y. Song, MD, Department of Radiation Oncology and Molecular Radiation Sciences, Johns Hopkins University School of Medicine
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
References:


