(UroToday.com) The 2023 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between June 2nd and June 6th was host to a prostate, testicular, and penile cancers oral abstract session. Following presentations by Dr. Olmos (CAPTURE 1 Cohort: Genetic mutations in mCRPC patients treated in the 1st line setting), Dr. Fizazi (TALAPRO-2), and Dr. Sandhu (LuPARP), Dr. VanderWeele delivered the discussant for these studies.
Dr. VanderWeele began by highlighting the following clinical questions that remain unanswered:
- Should we do molecular testing for all mCRPC patients?
- The answer appears to be clearly yes, based on the CAPTURE and TALAPRO-2 results (among other studies)
- Which genes matter?
- BRCA1/2 mutations appear to have significant prognostic and predictive values
- Should we skip genetic testing and give everyone a PARP inhibitor, as part of combination therapy in the 1st line setting?
Before diving deeper into each of the three studies, Dr. VanderWeele highlighted his take-home messages for each of the studies:
- CAPTURE:
- Homologous recombination repair (HRR) genes are important prognostic biomarkers, but BRCA1/2 are the most important
- Results of this study support genetic testing for all mCRPC patients
- TALAPRO-2:
- BRCA1/2 are excellent predictive biomarkers for PARP inhibitor response
- The trial population does not necessarily reflect current patient populations
- LuPARP:
- Intermittent olaparib plus LuPSMA appears to be safe in the short-term
- Questions regarding long-term safety and response rates/duration remain unanswered
CAPTURE included patients with mCRPC treated in the 1st line setting, using data from four prospective studies (5 cohorts), including PROREPAIR-B, PROSTAC (docetaxel and cabazitaxel), PROSABI (abiraterone acetate + prednisone), and PROSENZA (enzalutamide). This analysis included 729 patients, of whom 223 had tumors with HRR mutations. Analysis from this cohort demonstrated that BRCA1/2 patients had worse radiographic progression-free survival (rPFS), progression-free survival 2 (PFS2), and overall survival (OS).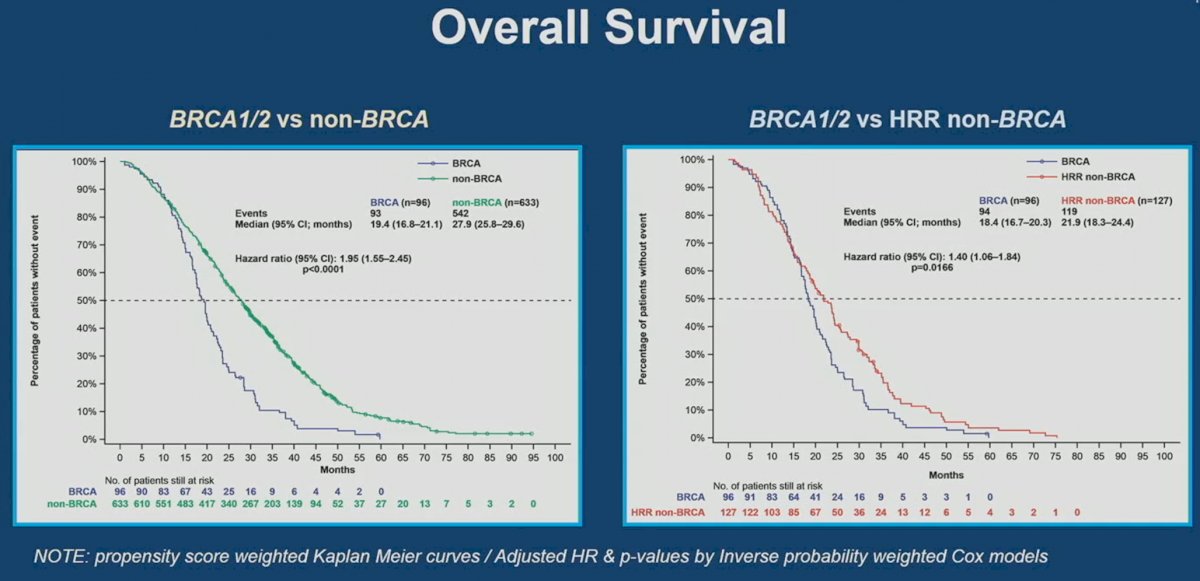
It appears that outcomes are worst for mCRPC patients with BRCA1/2 mutations, followed by HRR non-BRCA, the overall cohort, and then non-HRR mutated patients.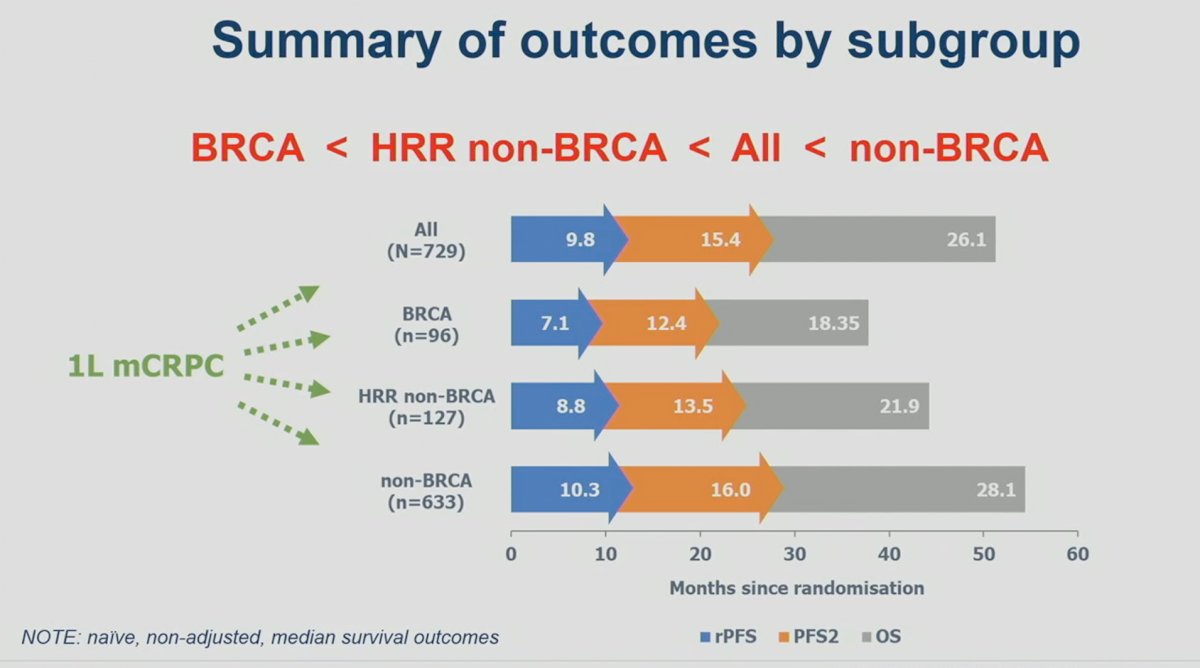
Significantly, within the BRCA1/2 cohort, it appears that rPFS, PFS2, and OS outcomes were similar irrespective of whether the mutations were mono- or bi-allelic or germline versus somatic.
Next, Dr.VanderWeele discussed the latest results from Cohort 2 (HRR mutated) of TALAPRO-2, which was concurrently published in The Lancet on the day of its ASCO presentation. At a median follow-up of 16.8-17.5 months, the combination of talazoparib plus enzalutamide, versus placebo and enzalutamide, was associated with significant improvements in rPFS in mCRPC patients with HRR mutations treated in the 1st line setting (HR: 0.45, 95% CI: 0.33 – 0.61, p<0.001).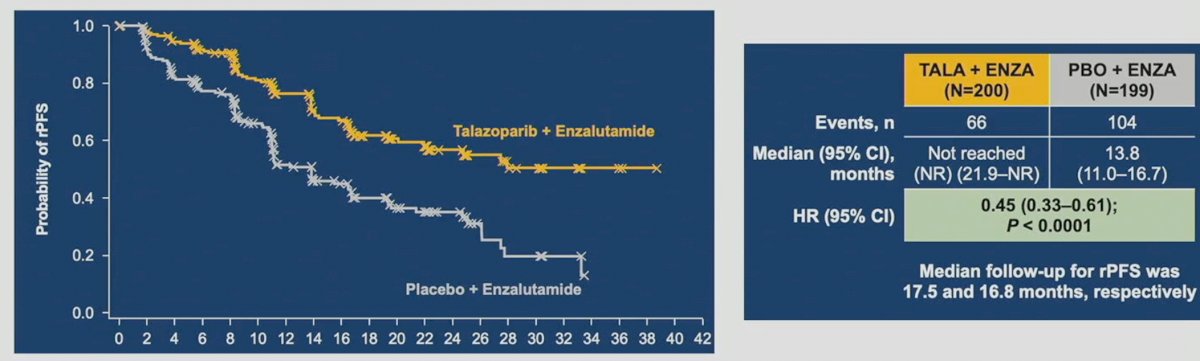
How do these results compare to those previously reported from PROpel2 and MAGNITUDE?3 As summarized in the table below, the rPFS benefit with combination talazoparib/enzalutamide, versus placebo/enzalutamide, appears to be consistent with that observed in the PROpel trial of abiraterone + olaparib (HR: 0.50) and better than that seen with the combination of abiraterone + niraparib (HR: 0.73). Similar to PROpel and MAGNITUDE, the magnitude of benefit appears to be greatest in the BRCA1/2 cohort with an 80% decrease in the rPFS hazard in TALAPRO-2. As, such it appears that BRCA1/2 mutations are excellent predictive biomarkers for PARP inhibitors, and the efficacy of combination enzalutamide + talazoparib is at least as good as that observed with other androgen receptor signaling inhibitor (ARSI) + PARP inhibitor combinations.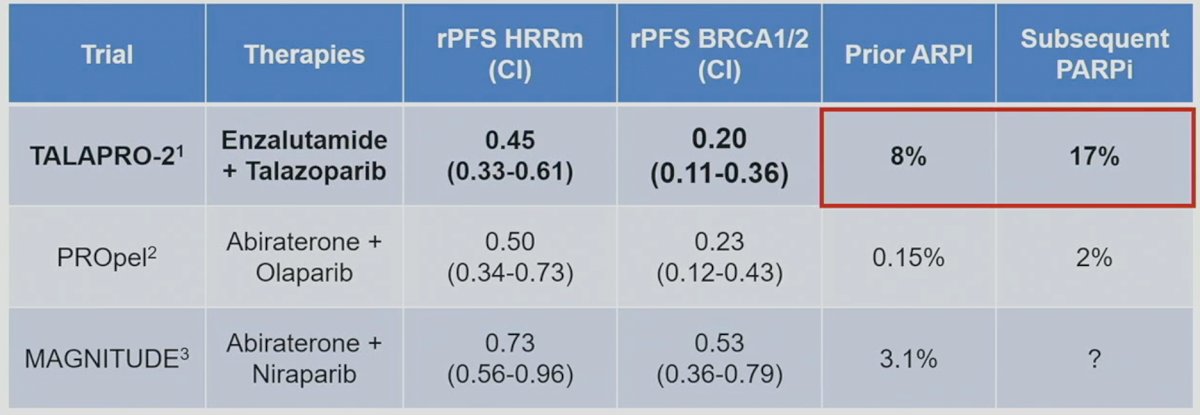
However, similar to PROpel and MAGNITUDE, Dr. VanderWeele argued that these populations do not necessarily reflect what we see in our practices today. With the emergence of early treatment intensification with doublet and triplet therapy regimens in the mHSPC disease space, many patients are already exposed to ARSIs and docetaxel prior to progressing to mCRPC. Only 8% of patients in TALAPRO-2 had prior ARSI use. Furthermore, only 17% of patients in the control arm received subsequent PARP inhibitors. As such, TALAPRO-2 studies the benefit of PARP inhibitors use (yes versus none), but not necessarily their combinatory or sequential benefits, as witnessed by the low uptake of subsequent PARP inhibitors in the control arm following disease progression. This is in contrast to the BRCAaway trial, a biomarker selected, randomized, open-label, multicenter phase 2 trial, which tests the combinatory and sequential effects of abiraterone (ARSI) and/or olaparib (PARP inhibitor) in mCRPC patients.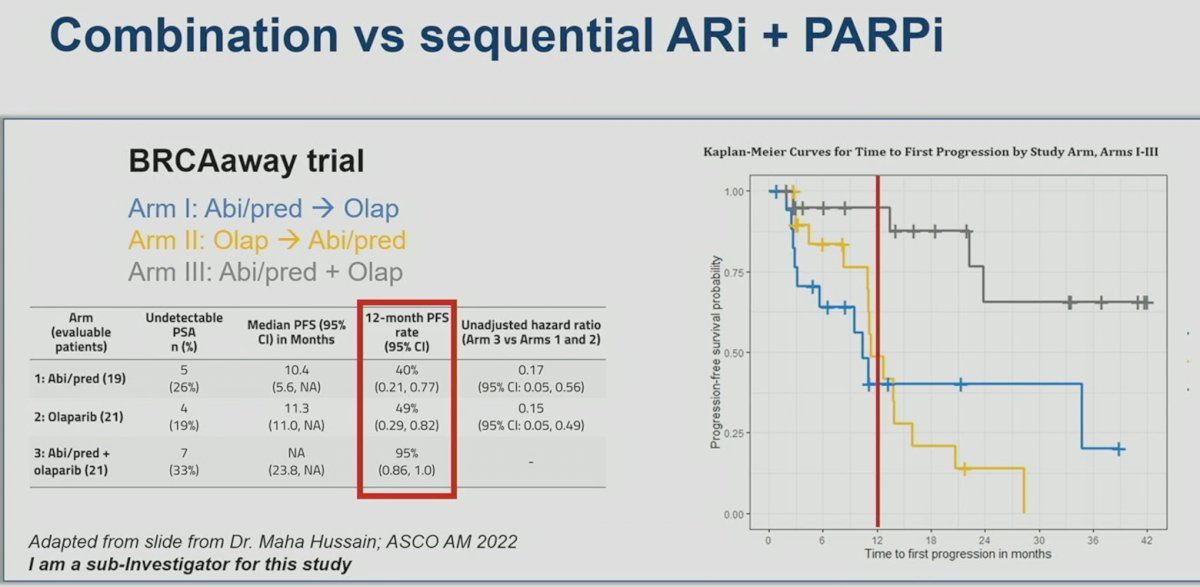
Current trials evaluating the combination of PARP inhibitors and ARSIs for earlier stage disease are summarized in the table below: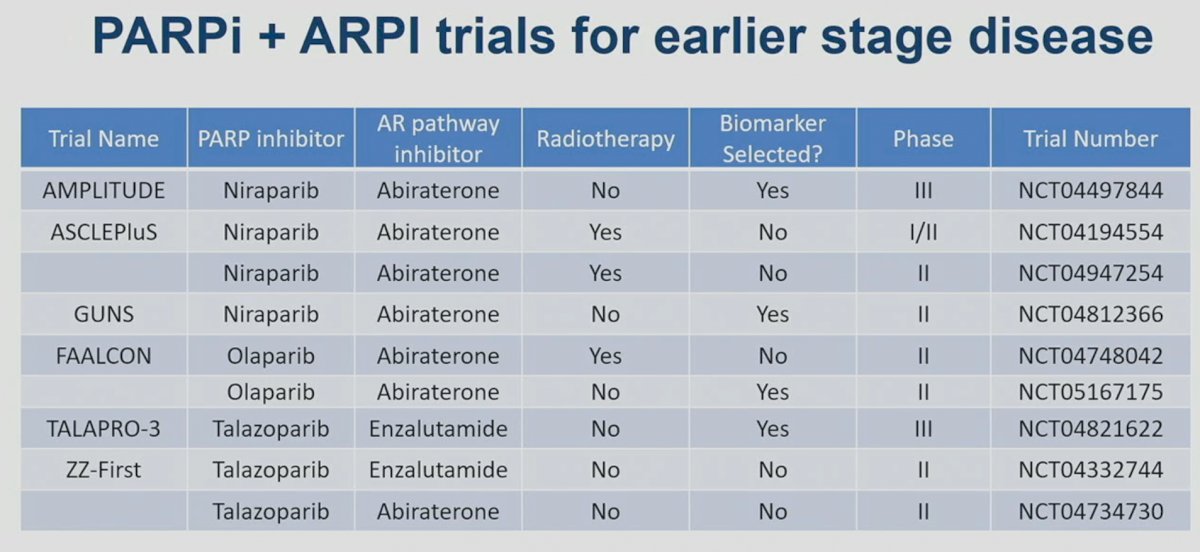
Finally, Dr. VanderWeele discussed the LuPARP trail, presented by Dr. Sandhu. LuPARP is a phase 1 trial of 48 patients with mCRPC, with all eligible patients having received a prior ARSI and docetaxel (i.e., ≥3L setting). All patients underwent a 68Ga-PSMA-11 plus an FDG-PET/CT with the following inclusion criteria:
- PSMA SUVmax >15 at any site
- SUVmax >10 at other sites
- No FDG discordance
This study followed a 3+3 dose escalation design. In cohorts 1-6, the dose of olaparib was sequentially increased from 50 mg to 300 mg on days 2-15 (day 0: day of LuPSMA administration). In cohorts, 7 to 9, the timing of olaparib administration (days -4 to 14 and -4 to 18), along with the dose of olaparib, were sequentially varied.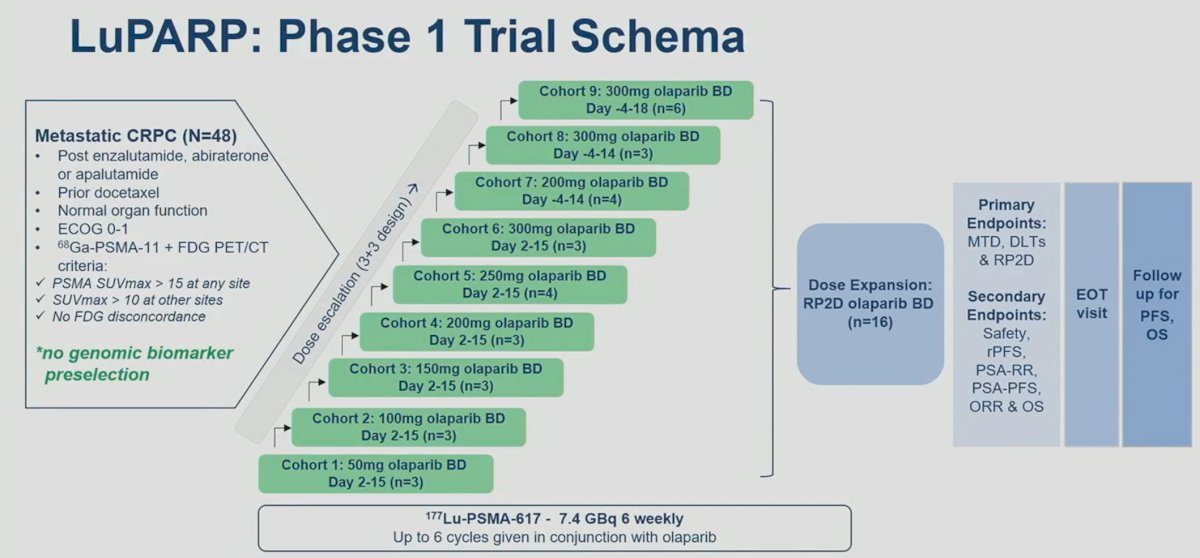
177Lu-PSMA-617 was administered at a dose of 7.4 GBq 6 weekly for 6 cycles. Olaparib was concurrently administered at a dose of 50 to 300 mg twice daily on days 2 to 15, -4 to 14, or -4 to 18 of each 6-week cycle, depending on the cohort assignment.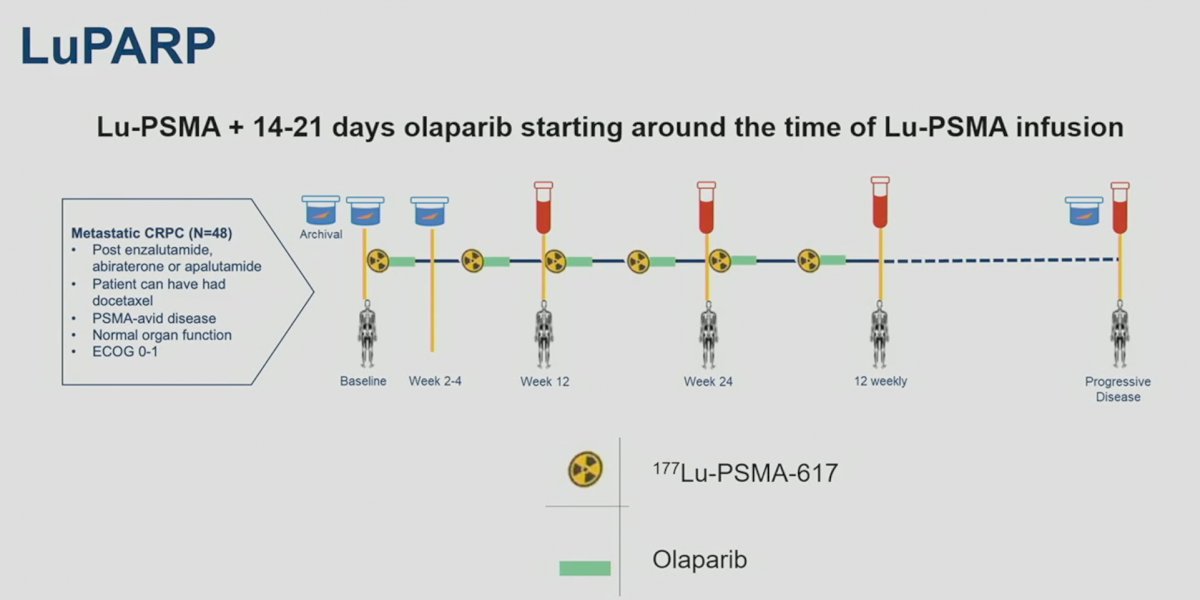
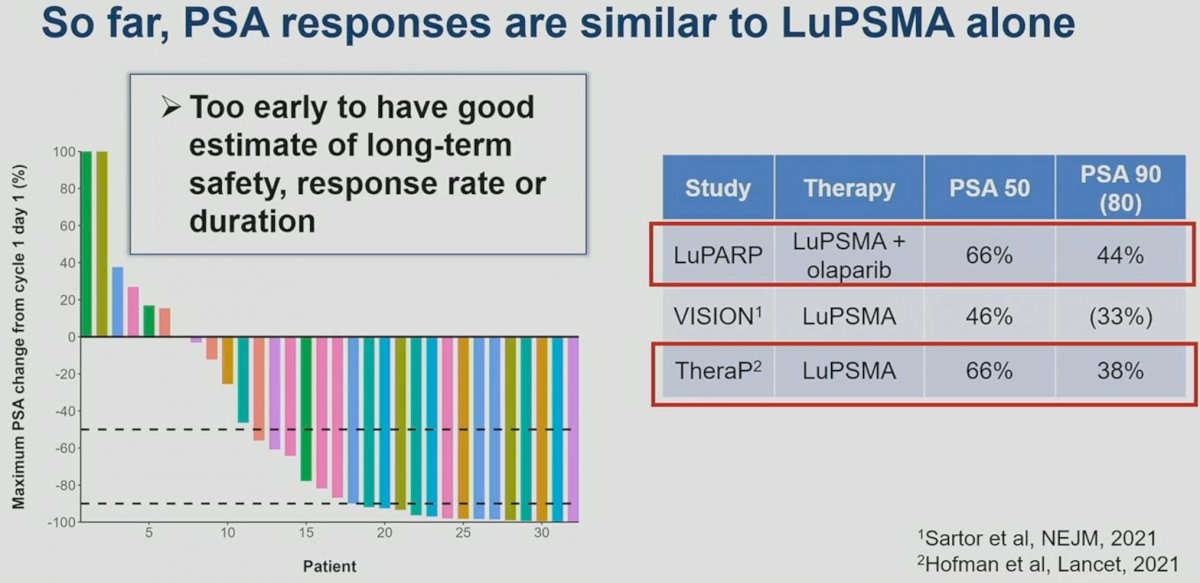
The combination of 177Lu-PSMA-617 and olaparib appears to be safe in the short-term, with no dose-limiting toxicities reported across the assessed dose levels. There were no grade 4 adverse events (AEs). One treatment-related serious AE occurred (febrile neutropenia). Dose delay due to hematological toxicity occurred in 3 (9%) patients (cohorts 2, 5, and 6). Dose reduction was required in 4 patients (12%), 3 due to hematological toxicity and 1 due to xerostomia.
Early efficacy outcomes suggest that this combination has PSA50 (66%) and PSA90 (44%) rates similar to those reported in TheraP (66% and 38%), but better than those seen in VISION (46% and 33%).4,5
In addition to LuPARP, other trials are evaluating LuPSMA combination regimens: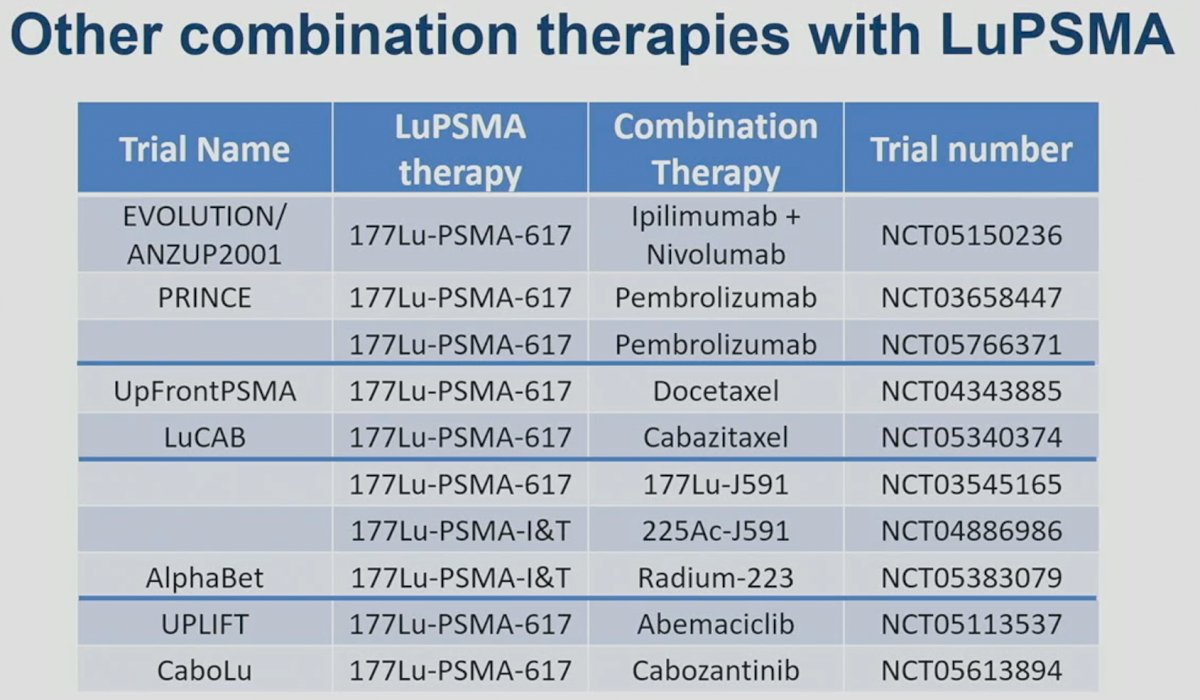
Presented by: Dr. David James VanderWeele, MD, PhD, Associate Professor, Division of Hematology and Oncology, Department of Medicine, Northwestern University, Chicago, IL
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
References:- Agarwal N, Azad A, Carles J, et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): a randomised, placebo-controlled, phase 3 trial. The Lancet. 2023.
- Clarke NW, Armstrong AJ, Thiery-Vuillemin A, et al. Abiraterone and Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2022; 1(9).
- Chi KN, Rathkopf DE, Smith MR, et al. Niraparib and Abiraterone Acetate for Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol. 2023; JCO2201649.
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med, 2021;385(12):1091-1103.
- Hofman MS, Emmett L, Sandhu S, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomized, open-label, phase 2 trial. Lancet, 2021;397(10276):797-804.


