(UroToday.com) At the 2023 ASCO annual meeting, Dr. Tanya Dorff presents the final results from a phase I study of PSCA-targeted chimeric antigen receptor (CAR) T cells in patients with metastatic castration resistant prostate cancer (mCRPC).
Chimeric antigen receptor (CAR)-engineered T cell therapies are being pursued for the treatment of many malignancies, including mCRPC. Prostate Stem Cell Antigen (PSCA) is highly expressed on the surface membrane in mCRPC but has limited expression on normal tissues, making it an ideal target for therapy.
In this study, the authors note that they are completing a phase I study of PSCA-targeted 4-1BB-co-stimulated CAR T cell therapy in mCRPC.
Eligible patients include adult patients with metastatic castration resistant prostate cancer whose cancer has progressed after at least one androgen receptor targeted agent. Prior taxane chemotherapy was allowed but not required; there was no limit on prior lines of therapy.
CAR T cells were manufactured at City of Hope’s cGMP facility.
As for study design, they followed target equivalence range design (range 0.2-0.35) and too toxic level of 0.51 with enrollment of participants in cohorts of 3. The plan began was a dose of 100 Million (M) CAR T cells without lymphodepletion (LD), then added LD to 100M (DL2) and a plan to escalate to 300M and LD.
The primary objective was to define the dose limiting toxicities (DLT) as well as to describe preliminary bioactivity and activity. CAR T persistence and cytokine levels were evaluated by flow cytometry and Luminex. Circulating tumor cells (CTC) were measured by an unselected assay (Kuhn).
14 pts were treated, demographics stratified by treatment dose is seen below:
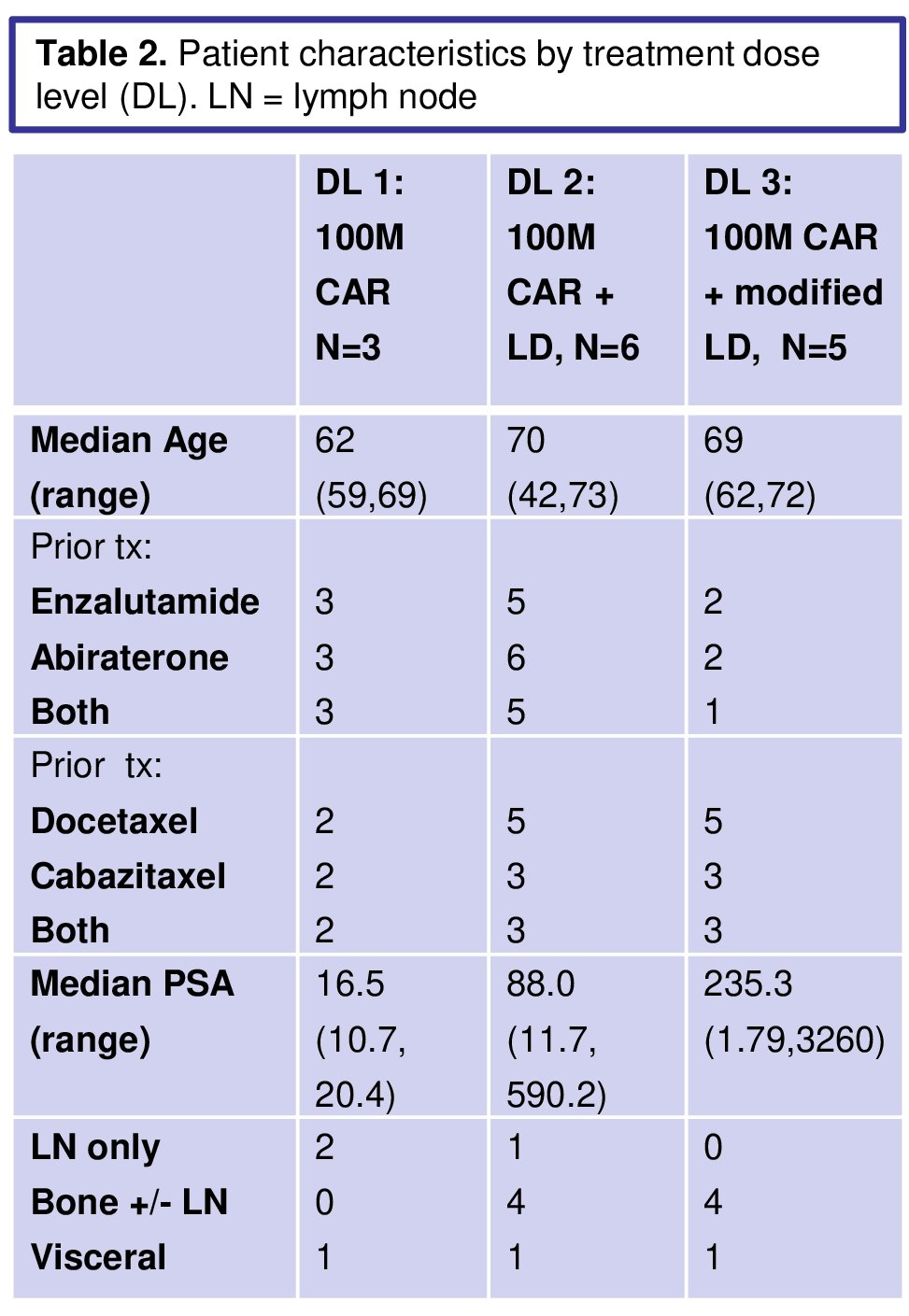
79% of screened pts had PSCA expression >2+ in >80% of cancer cells.
 \\
\\
Two of 6 pts encountered DLT (grade 3 cystitis) at 100M + lymphodepletion (LD) chemotherapy. The protocol was amended to reduce the LD dose to 300 mg/m2 cyclophosphamide D1-3. No DLTs occurred in 5 pts treated in the modified LD 100M cohort.
Based on city, activity, at preclinical results, they decided not to dose escalate but rather to open a phase 1B with a total dose of CAR T (50M infused up to 6 times) and radiation to enhance the tumor microenvironment.
Cytokine release syndrome (CRS), best response by RECIST, CAR T cell expansion and persistence are presented by dose level in the table.
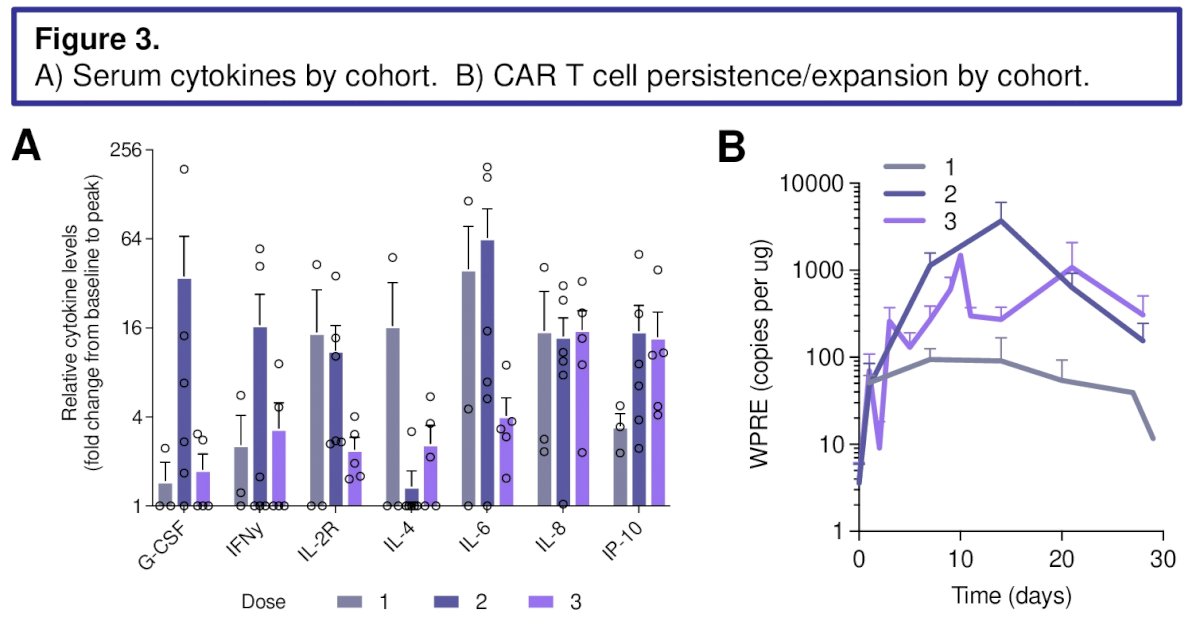
CAR T expansion was greater with LD than without. PSA declines were seen, as well as radiographic improvement, though RECIST response was limited to stable disease (SD) by concurrent bone metastases.
PSA decline is seen below and is modest.

Tumor response is summarized below:

Cytokine peaks were higher in patients with anti-tumor effect noted. CTC decreases in both marrow and peripheral blood were seen, associated with PSA declines.
Based on this data, Dr. Dorff and colleagues report that PSCA-CAR T cells have anti-cancer activity in mCRPC with DLT of cystitis. PSCA is rest in mCRPC, with >80% screened patient having at least 2+ tissue expression. LD was required for greater expansion and activity; lower dose LD improved toxicity without clear negative impact on expansion. Ultimately, they felt that this represented a safe and feasible option – and such have already opened a phase 1b trial to determine treatment dosage.
Clinical trial information: NCT03873805.
Presented by: Tanya B. Dorff, MD., is an associate clinical professor in the Department of Medical Oncology & Therapeutics Research and serves as head of the genitourinary cancers program at City of Hope.
Written by: Thenappan (Thenu) Chandrasekar, MD – Urologic Oncologist, Associate Professor of Urology, University of California, Davis, @tchandra_uromd @UCDavisUrology on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.


