(UroToday.com) The 2023 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between June 2nd and June 6th was host to a prostate, testicular, and penile cancers poster discussion session. Dr. Andrew Armstrong presented the health-related quality of life (HRQoL) and pain outcomes for mCRPC patients receiving abiraterone + olaparib versus abiraterone + placebo in the phase III PROpel trial.
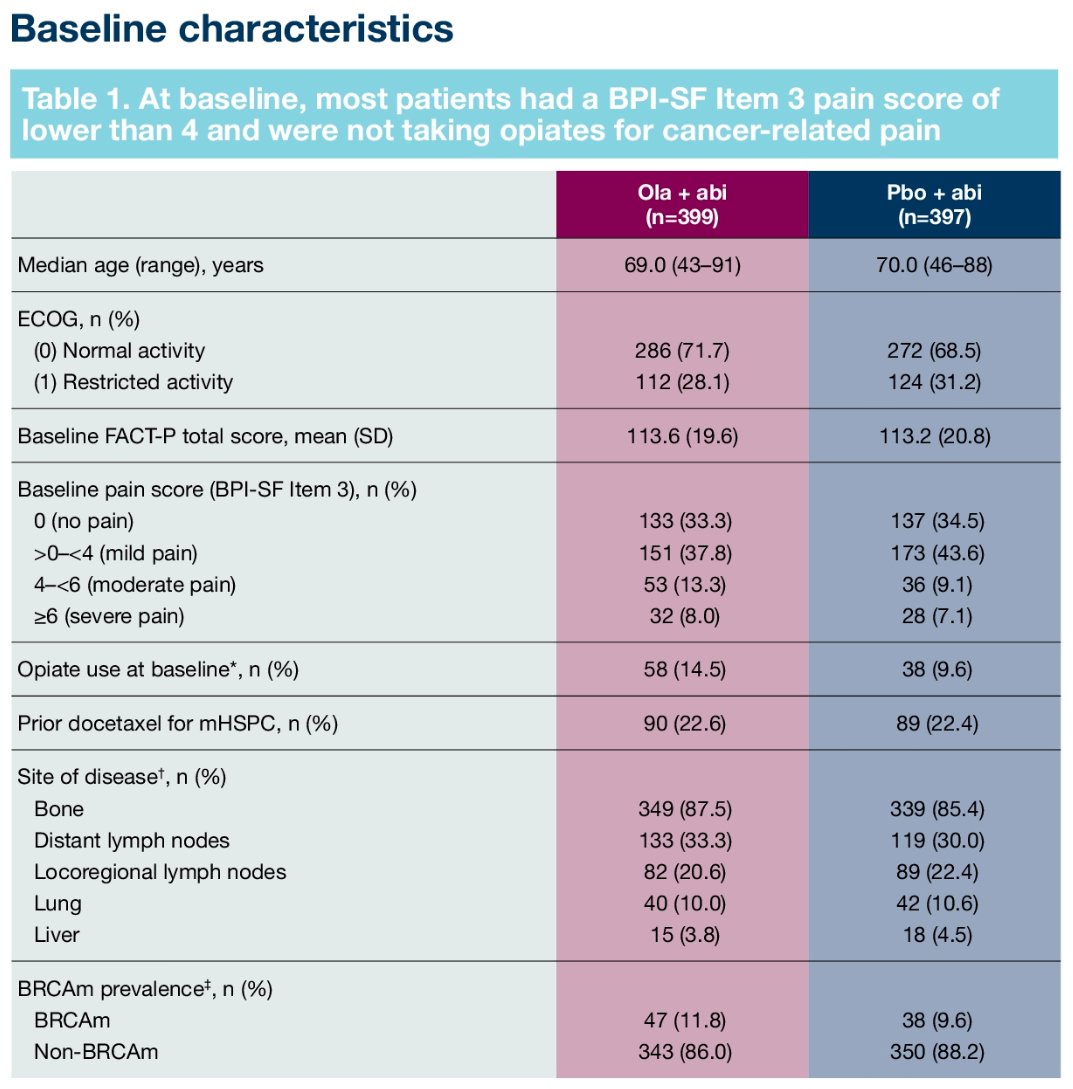
PROpel is a global, randomized, double-blind phase 3 trial of abiraterone and olaparib versus abiraterone and placebo in patients with mCRPC treated in the first-line setting. Patients in PROpel were enrolled irrespective of HRRm status. Patients were randomized (1:1) to receive abiraterone (1000 mg once daily) plus prednisone/prednisolone with either full dose olaparib (300 mg twice daily) or placebo. At a median follow-up of 19.4 months, the combination of olaparib/abiraterone significantly improved median investigator-assessed rPFS from 16.6 to 24.8 months (HR: 0.66, 95% CI: 0.54 – 0.81, p<0.001). rPFS benefits were observed irrespective of HRRm status, although, as expected, the magnitude of effect was higher in the HRRm (HR: 0.50, 95% CI: 0.34 – 0.73) versus non-HRRm patients (HR: 0.76, 95% CO: 0.60 – 0.97). To date, no significant OS benefits have been observed in the overall cohort with this combination.1
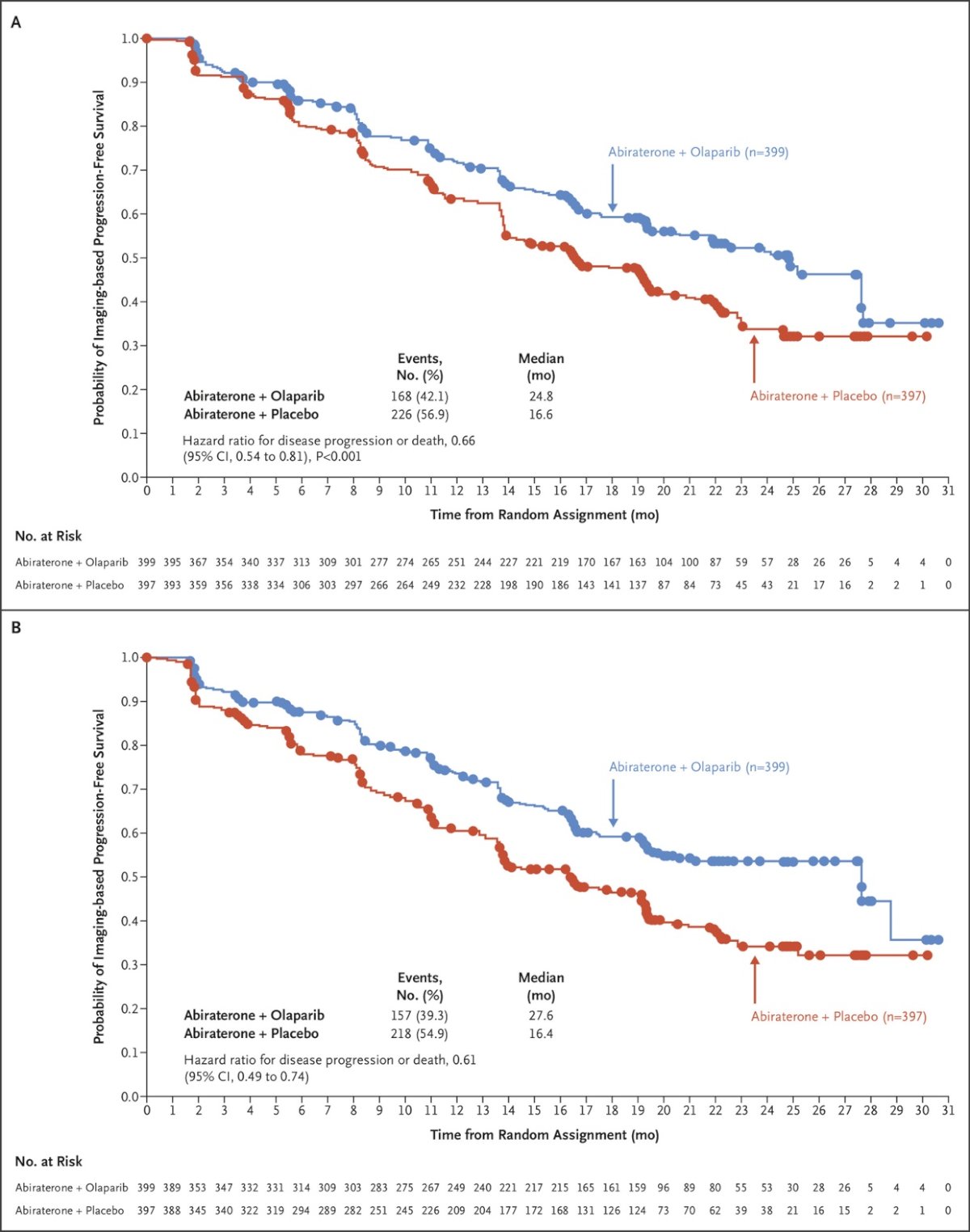
In the initial report, there were no significant differences in HRQoL outcomes between the two arms, as evaluated by the Functional Assessment of Cancer Therapy-Prostate (FACT-P) questionnaire. In this study, the investigators report HRQoL data at the final pre-specific overall survival cut-off date of October 12, 2022.
The investigators evaluated between treatment arms differences in:
- HRQoL:
- FACT-P total and subscale scores
- BPI-SF pain severity
- Pain interference
- Worst pain score
- Time to pain progression, based on the Brief Pain Inventory-Short Form (BPI-SF) Item 3 ‘worst pain in 24 hours’ and opiate analgesic use score
- Time to first symptomatic skeletal-related event, defined as time to use of therapy to prevent/relieve skeletal symptoms, new bone fractures, spinal compression, or surgery for bone metastases
Given the repeated measurements nature in the same individuals evaluated longitudinally, a mixed models approach was employed.
The questionnaire completion rate ranged between 70% and 94%, as summarized below:

No significant differences were observed in FACT-P assessed HRQoL scores between the two arms:

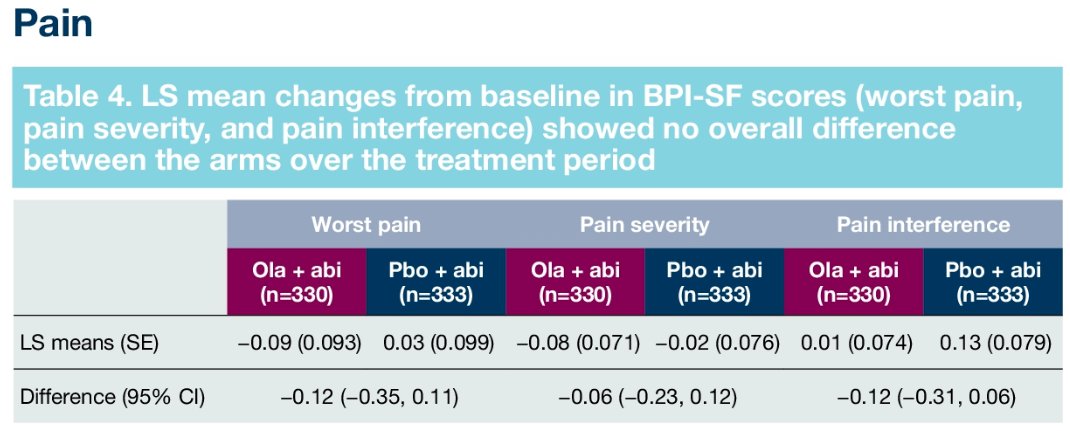
Similarly, pain scores and time-to-pain progression rates were non-significantly different between the two arms:
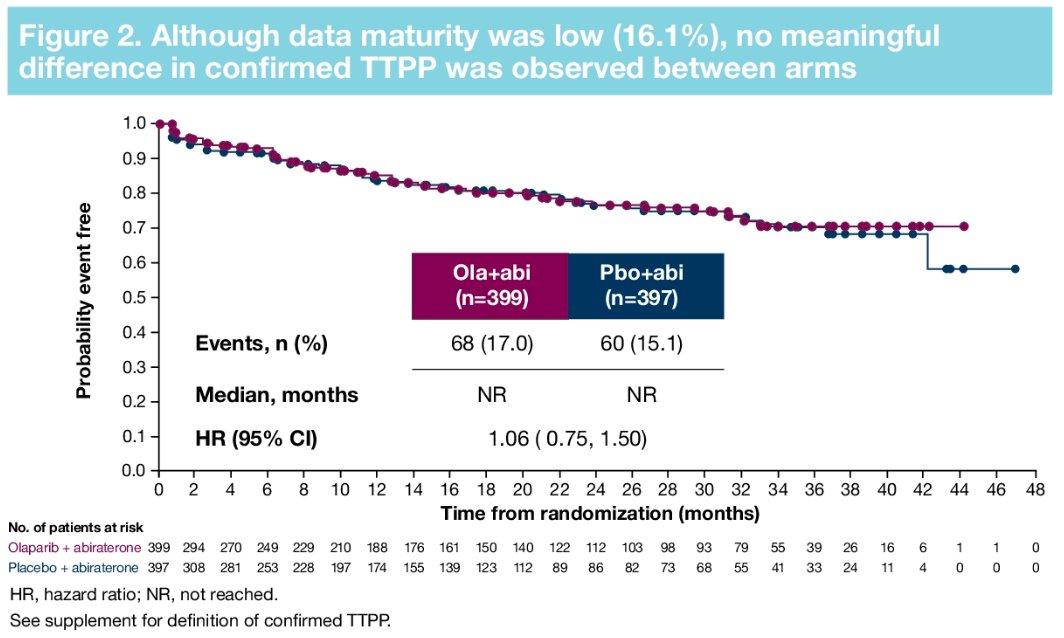
Furthermore, there were no significant differences in time to first symptomatic skeletal-related event (HR: 0.82, 95% CI: 0.55 – 1.22) or time to opiate use (HR: 1.21, 95% CI: 0.82 – 1.79). in the overall cohort.
In conclusion, it appears that the use of combination abiraterone plus olaparib, compared to abiraterone alone, is not associated with worsening of HRQoL outcomes, as assessed by the FACT-P total and subscale scores, BPI-SF domain, and worst pain scores. This suggests that patients can derive rPFS benefits from this combination while maintaining a similar HRQoL.
Presented by: Andrew J. Armstrong, MD, Professor, Department of Medicine, Duke Cancer Institute Center for Prostate and Urologic Cancer, Duke University, Durham, NC
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
References:

