(UroToday.com) The 2023 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between June 2nd and June 6th was host to a prostate, testicular, and penile cancers poster session. Dr. Chinmay Jani presented an update on COMRADE, a phase 1/2 study evaluating the combination of olaparib and Radium-223 in mCRPC patients with bone metastases.
Radium-223 dichloride (radium-223) is a targeted alpha emitter that selectively binds to areas of increased bone turnover in bone metastases and emits high-energy alpha particles of short-range (<100 μm), resulting in double-stranded DNA breaks. This results in a potent and highly localized cytotoxic effect in the target areas. Radium-223 was FDA approved in 2013 based on the results of the ALSYMPCA, a phase 3, randomized, double-blind, placebo-controlled trial that randomized 921 patients in a 2:1 fashion to receive six injections of radium-223 (at a dose of 50 kBq per kilogram of body weight intravenously) or matching placebo. Patients receiving radium-223 had a significantly improved median overall survival (OS) of 14 versus 11.2 months (HR: 0.70, 95% CI: 0.55 – 0.88).1
Poly (adenosine diphosphate-ribose) polymerase inhibitors (PARPi) prevent the repair of DNA single-stranded breaks and promote their conversion to double-stranded breaks. These agents have demonstrated OS benefits in the 2nd line setting for HRR-mutated (HRRm) mCRPC patients who had progressed on prior androgen receptor signaling inhibitors (ARSI),2-4 and combination with ARSIs in the first-line mCRPC treatment setting.5-7
Pre-clinical models have suggested that PARPi may work as radiosensitizing agents. As such, the investigators designed COMRADE as a phase 1/2 trial to identify the recommended phase 2 dose of olaparib and radium-223 and test the hypothesis that radium-223 + olaparib demonstrates anti-tumor activity in mCRPC patients, irrespective of homologous recombination repair (HRR) mutational status.
COMRADE (NCT03317392) is an open label, multicenter, phase 1/2 trial that will evaluate the dosing, safety, and efficacy of olaparib plus radium-223 in men with mCRPC and bone metastases. This study includes mCRPC patients with any number of prior therapy lines for mCRPC, ≥2 bone metastases by imaging, and at least one lesion which has not been treated with prior radiation. Patients with visceral metastases or malignant lymphadenopathy (> 4 cm in short diameter) were excluded.
COMRADE is split into two phases components:
- Phase 1: Uses a 3+3 dose escalation design to determine the olaparib recommended phase 2 dose + fixed dose Radium-223 (55 kBq/kg IV every 4 weeks x 6).
- Phase 1 has completed accrual (n=12) and demonstrated that the recommended phase 2 dose of olaparib is 200 mg orally twice daily (300 mg in PROpel) when combined with standard dose radium-223.
- At full dose of 300 mg orally twice daily, 5/6 patients required a dose reduction
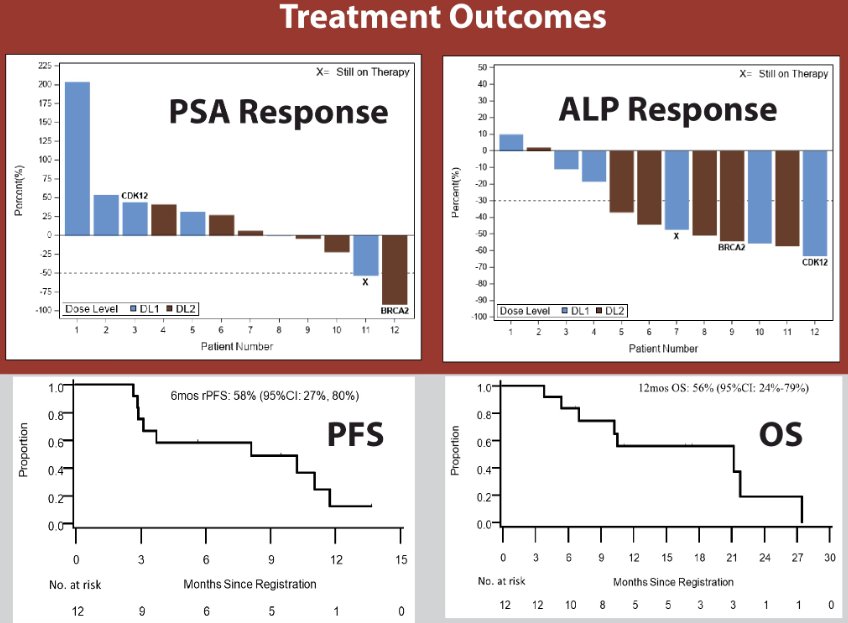
- Early treatment outcomes from phase 1 are as follows:
- Phase 1 has completed accrual (n=12) and demonstrated that the recommended phase 2 dose of olaparib is 200 mg orally twice daily (300 mg in PROpel) when combined with standard dose radium-223.
- Phase 2: Open-label, randomized trial (1:1) evaluating olaparib + radium-223 compared to radium-223 alone (activated January 2021)
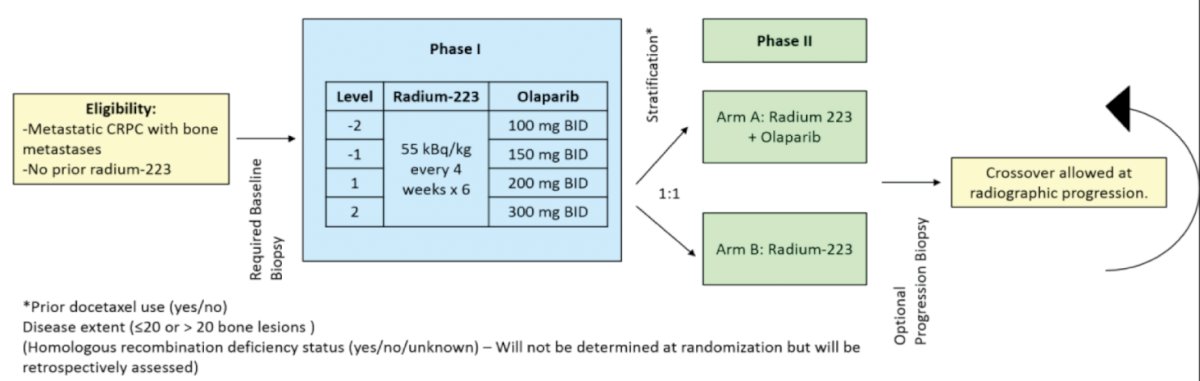
The primary endpoint for the phase 2 component was radiographic progression-free survival (rPFS), assessed per PCWG3 guidelines for bone metastases and RECIST v1.1 for non-bone disease. Key secondary endpoints include:
- Time to PSA progression
- PSA response
- Time to subsequent therapy
- Time to first skeletal event
- OS
HRR mutational status is determined using the Oncopanel assay. A key secondary endpoint will be to evaluate rPFS in biomarker positive and negative patients. Exploratory endpoints will include whole exome and tissue sequencing of baseline biopsy tissue, assessment of RAD51 IHC and correlation with outcomes, ctDNA assessment and correlation with responses, evaluation of changes in the tumor immune microenvironment, and quality of life assessment. The target accrual is 133 patients.
Presented by: Chinmay Jani, MBBS, Chief Medical Resident, Mount Auburn Hospital, Harvard Medical School, Boston, MA
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
References:
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213-223.
- De Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med 2020; 382(22): 2091-102.
- Abida W, Patnaik A, Campbell D, et al. Rucaparib in Men With Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J Clin Oncol 2020; 38(32): 3763-72.
- Fizazi K, Piulats JM, Reaume MN, et al. Rucaparib or Physician’s Choice in Metastatic Prostate Cancer. N Engl J Med 2023; 388: 719-32.
- Clarke NW, Armstrong AJ, Thiery-Vuillemin A, et al. Final overall survival (OS) in PROpel: abiraterone (abi) and olaparib (ola) versus abiraterone and placebo (pbo) as first-line (1L) therapy for metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol 2023; 41(Suppl 6; abstr LBA16).
- Chi KN, Rathkopf DE, Smith MR, et al. Niraparib and Abiraterone Acetate for Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol 2023; JCO2201649.
- Agarwal N, Azad A, Carles J, et al. Abiraterone and Olaparib for Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol 2023; 41 (Suppl 6; abstr LBA17).


