(UroToday.com) The 2023 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between June 2nd and June 6th was host to a prostate, testicular, and penile cancers poster session. Dr. Anis Hamid presented analysis from the LuPARP trial evaluating circulating tumor cells (CTCs) in patients with mCRPC treated with olaparib plus 177Lu-PSMA-617.
LuPARP (NCT03874884) is a phase 1 single arm trial evaluating the safety and efficacy of combination 177Lu-PSMA plus olaparib (PARP inhibitor) in mCRPC patients who have progressed following prior chemotherapy and androgen receptor signaling inhibitor (ARSI) use. In this analysis of the LuPARP trial, Dr. Hamid and colleagues aimed to identify potential prognostic and predictive biomarkers from serial CTC profiling.
This trial included a total of 26 patients with high PSMA expression on PSMA-PET/CT. These patients received 177Lu-PSMA-617 every 6 weeks together with an escalating dose-schedule of olaparib (50mg twice daily up to 300mg twice daily, days 2 to 14 or days -4 to 14) for up to 6 cycles.
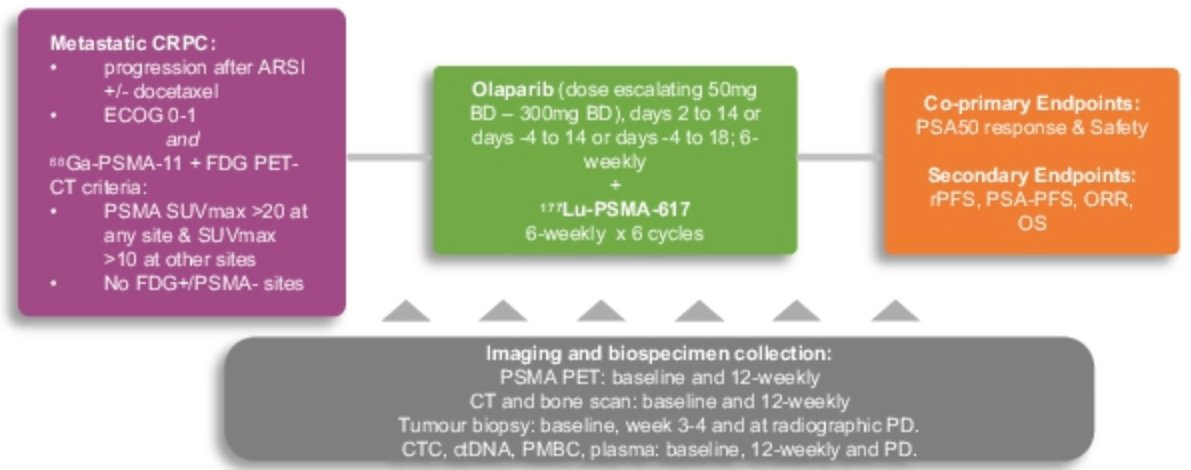
For CTC analysis, 10 mls of blood were collected at:
- Baseline
- Every 12 weeks for the first 48 weeks
- Every 24 weeks thereafter or at disease progression
CK+, CD45-, and DAPI+ CTCs were enumerated from 3 mls of blood and immunoassayed for PSMA expression using the Epic Sciences platform. The investigators evaluated the correlations between PSA 50 response (PSA reduction of ≥ 50%) and both total and PSMA+ CTC counts. Low pass whole genome sequencing (lpWGS) was performed at baseline and longitudinally on CTC samples to identify copy number alterations.
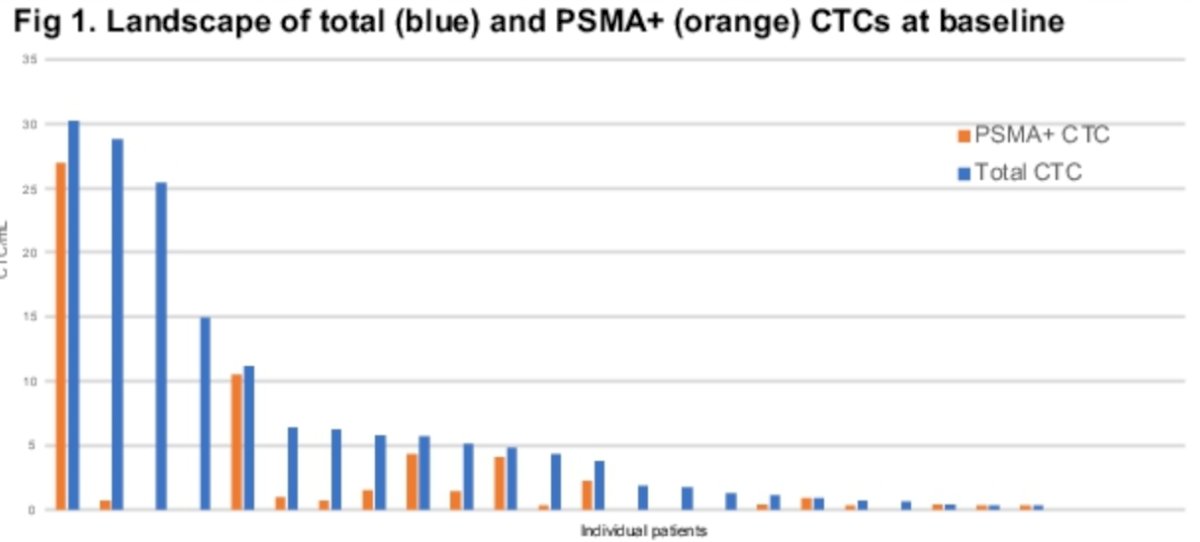
At baseline, 23 of the 26 patients (88%) had detectable CTCs (median 2.9 CTCs/ml, range: 0 to 30). Of these 23 patients with detectable CTCs, 17 (74%) had detectable PSMA+ CTCs, with a median of 0.9 CTCs/ml (range: 0.3 – 27), indicating heterogeneity of PSMA expression in CTCs.
The CTC positivity rates (% of cases ≥1 CTC) and PSMA positivity rates (% of cases ≥1 PSMA+ CTC) were as follows:
- Week 12: 57% and 38%, respectively
- Week 24: 58% and 36%
- Week 36: 75% and 22%
- Week 48: 36% and 50%
- At progression: 100% and 56%
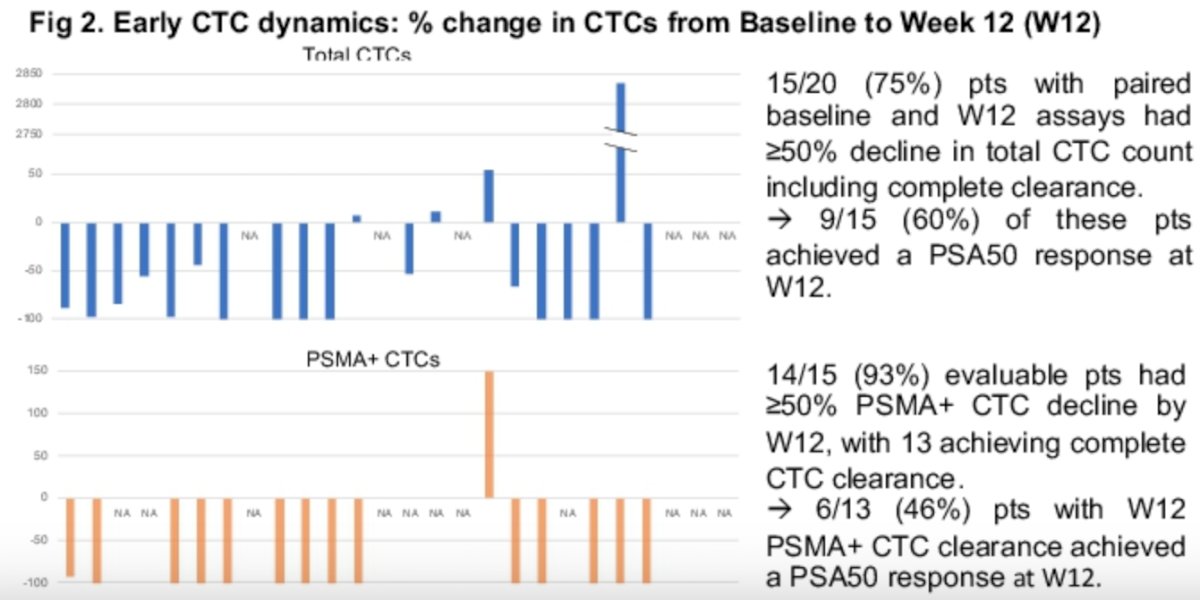
Next the investigators evaluated whether there was an association between changes in total CTC count and PSA50 responses. Fifteen of 20 (75%) evaluable patients with paired baseline and week 12 CTCs had a ≥50% decline in total CTC count, of which 9/15 (60%) pts also achieved a PSA50 response. Five of these 9 (56%) pts had 100% CTC clearance at week 12.
In terms of PSMA+ CTCs, 14/15 (93%) evaluable patients had ≥50% PSMA+ CTC decline with 13 pts (93%) achieving complete CTC clearance by week 12. 6/13 patients with complete PSMA+ CTC clearance at week 12 also achieved a PSA50 response.
Beyond PSMA expression, genomic heterogeneity was evident in baseline and on-treatment CTCs, including recurrent loss of PTEN, TP53, BRCA2, ATM, and RB1, and gain of AR and MYC. lpWGS in 10 baseline samples identified 5 cases with BRCA2 loss and 5 cases with ATM loss. Two pts with BRCA2 loss and 3 patients with ATM loss had a PSA50 response by week 12.
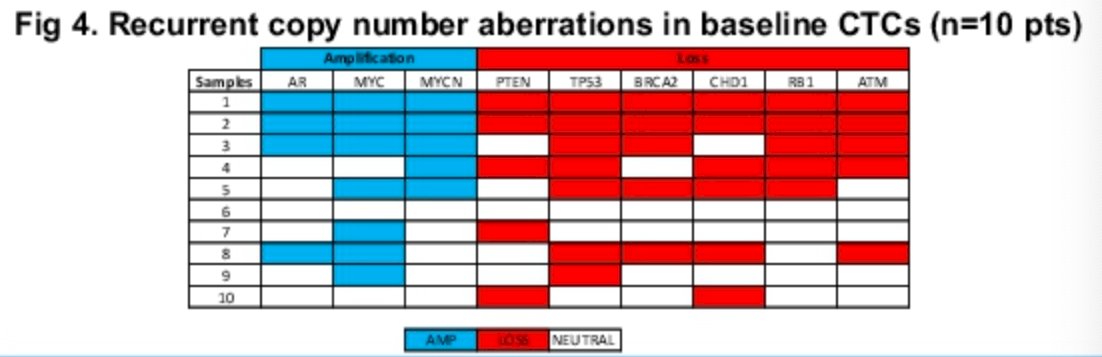
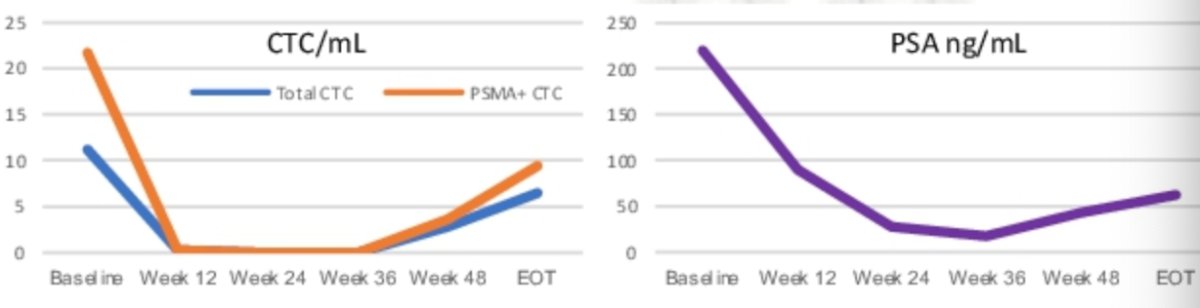
In summary, the investigators observed high rates of total and PSMA+ CTCs in PSMA-expressing mCRPC. Total and PSMA+ CTCs declined with combined olaparib and 177Lu-PSMA-617 treatment. Early declines in total and PSMA+ CTCs including 12 week CTC clearance paralleled PSA responses. Notably, total and PSMA+ CTCs rise again in the setting of disease progression. Dr. Hamid and colleagues concluded that serial CTC profiling may assist in tracking response to 177Lu-PSMA-617 therapy.
Presented by: Anis Hamid, MBBS, GU Oncology Research Fellow, Department of Medical Oncology, Peter MacCallum Cancer Centre and University of Melbourne, Melbourne, Victoria, Australia
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.


