(UroToday.com) The 2023 ASCO annual meeting included a prostate cancer session featuring a presentation by Wesley Armstrong discussing PSMA PET findings in an “EMBARK-like” cohort of patients with high-risk non-metastatic hormone-sensitive prostate cancer.
Non-metastatic hormone-sensitive prostate cancer is defined by rising PSA levels while naïve or responsive to ADT and without evidence of metastasis on conventional imaging. The EMBARK trial is a phase 3 randomized study evaluating the use of enzalutamide with or without concomitant leuprolide in non-metastatic castrate-sensitive prostate cancer patients. Eligibility status for the trial relied upon conventional imaging, which under-detects metastatic disease in comparison to PSMA-PET imaging. The aim of this post-hoc retrospective analysis was to describe the PSMA-PET findings in a patient population representative of the EMBARK trial.
This was a post-hoc, retrospective analysis of 4 prospective studies of PSMA PET conducted at UCLA from 2016 to 2021 that included patients with recurrent prostate cancer following radical prostatectomy, radiotherapy or salvage radiotherapy. Patients were included if they met eligibility for enrollment in the EMBARK trial at the time of their PSMA scan defined as the following: rising PSA at a level above 1.0 ng/mL (post radical prostatectomy and salvage radiotherapy) or 2.0 ng/mL > nadir (post-radiotherapy), PSA doubling time ≤ 9 months, and serum testosterone ≥ 150 ng/dL. Exclusion criteria constituted distant metastatic disease as assessed by radiographic imaging, prior hormonal therapy, or systemic therapy for prostate cancer. PSMA PET findings (PROMISE miTNM stage, number of lesions) were collected from the clinical imaging reports.
From 2,002 patients screened, 183 patients were included in the analysis. Median time from primary therapy to PSMA PET was 39 (2.1-261) months. Median pre-scan PSA levels and PSA doubling time in patients who underwent radical prostatectomy (n=92), definitive radiotherapy (n=39), post-radical prostatectomy salvage radiotherapy (n=52), and the overall cohort (n=183) were 2.4 ng/mL (IQR 1.4-4.8), 6.9 ng/mL (IQR 3.5-18.5), 2.7 ng/ml (IQR 1.7-5.3), 2.8 ng/mL (IQR 1.7-6.7), respectively, and 3.1 months (IQR 1.9-5.3), 3.2 months (IQR 2.1-4.8), 4 months (IQR 2.6-5.4), 3.6 months (IQR 1.9-5.3), respectively. A summary of the patient characteristics is as follows:
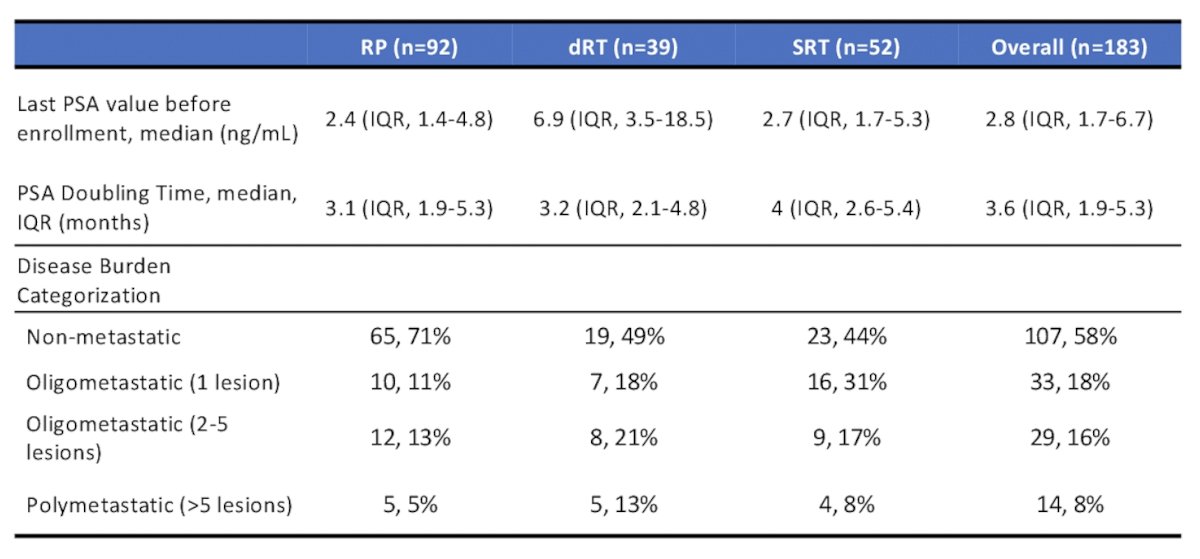
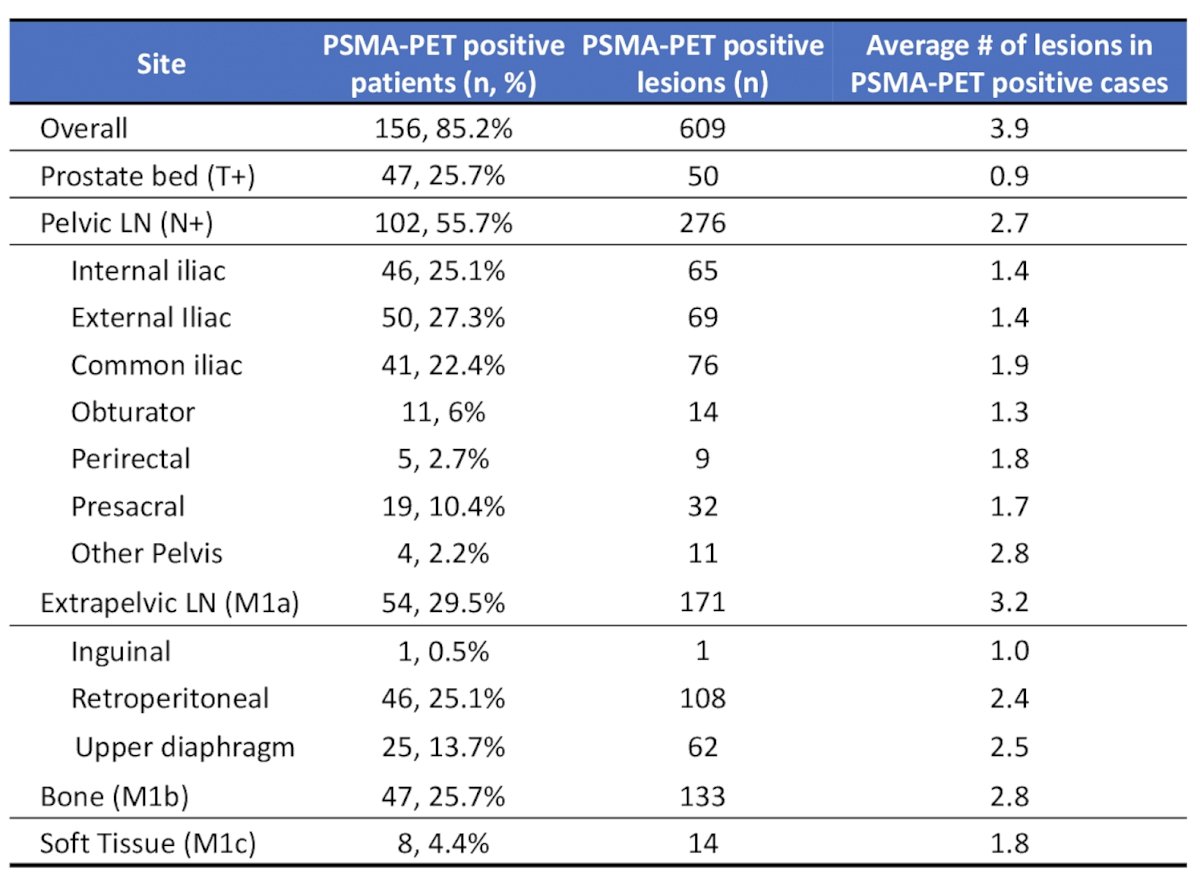
Overall, 85.2% of patients were PSMA PET positive, with 609 total PSMA PET positive lesions, and 3.9 mean number of lesions per case. The distribution of PSMA PET detected disease is as follows:
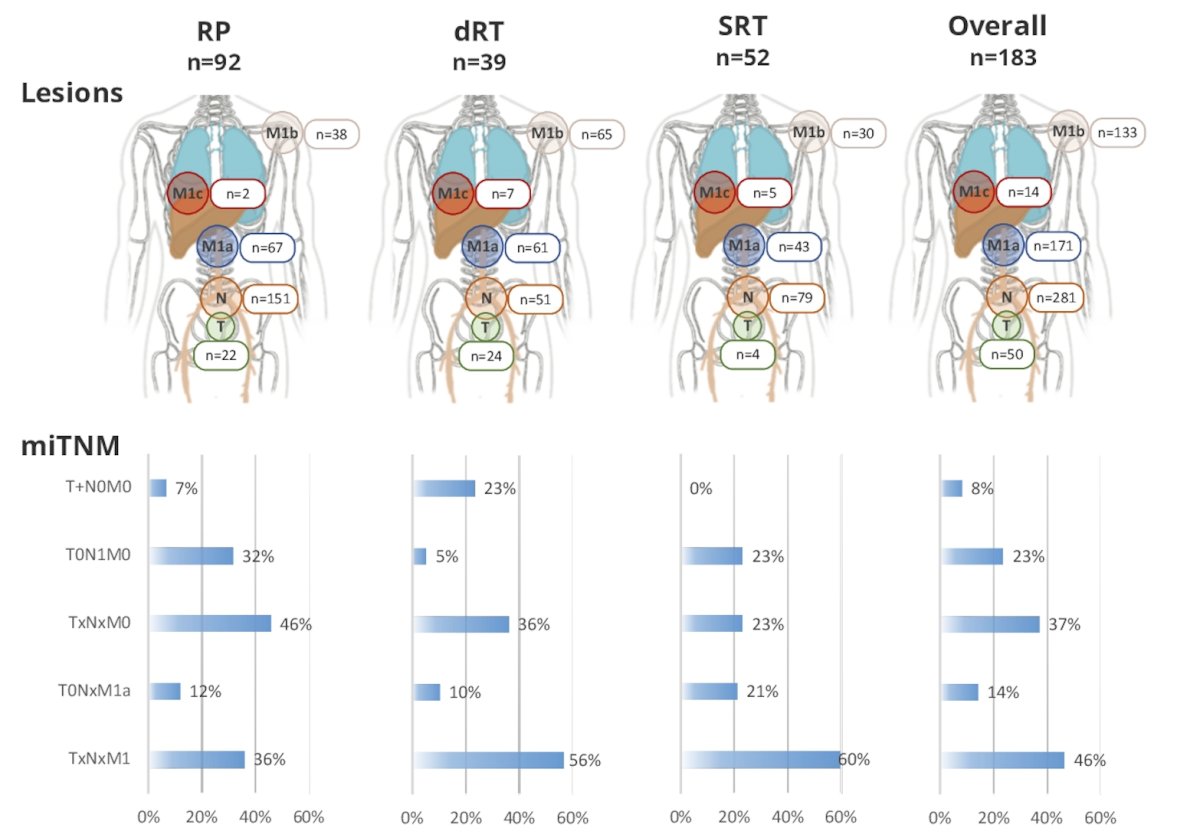
Finally, the following highlights the lesion and miTNM staging distributions by treatment modality:
Wesley Armstrong concluded his presentation discussing PSMA PET findings in an “EMBARK-like” cohort of patients with high-risk non-metastatic hormone-sensitive prostate cancer with the following take-home points:
- PSMA-PET detected M1 disease in 36%, 56%, 60%, and 46% of radical prostatectomy, definitive radiotherapy, salvage radiotherapy, and the overall cohort, respectively
- Oligometastatic disease was detected in 34% and polymetastatic disease (>5 M1 lesions) in 8% of patients
- Further studies are needed to assess its independent prognostic value and use for treatment guidance
Presented by: Wesley R. Armstrong, UCLA-Caltech Medical Scientist Training Program, Los Angeles, CA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter
during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.


