(UroToday.com) The 2023 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between June 2nd and June 6th was host to a kidney and bladder cancers poster session. Dr. Katy Beckermann, MD, PhD presented the results of a phase II trial evaluating batiraxcept (AVB-S6-500), an AXL inhibitor, as monotherapy, in combination with cabozantinib alone, and in combination with cabozantinib plus nivolumab in patients with advanced clear cell renal cell carcinoma (ccRCC).
Pre-clinical models have demonstrated that AXL, a receptor tyrosine kinase, is up-regulated by hypoxia-inducible factor-1 (HIF-1) signaling in VHL-deficient tumor cells, playing a critical role in metastasis and resistance to VEGF-targeted therapies. Batiraxcept is a recombinant fusion protein containing an extracellular region of AXL combined with the human immunoglobulin G1 heavy chain (Fc) and has demonstrated potent and specific AXL inhibition through competitive binding of its ligand GAS6. A prior Phase 1b study presented at ASCO GU 2023 showed promising outcomes for batiraxcept in combination with cabozantinib in patients who had failed prior 1st line therapy.1

This phase II trial (NCT04300140) tested batiraxcept at a dose of 15 mg/kg every 2 weeks in 3 distinct cohorts:
- Cohort 1: Batiraxcept monotherapy in patients with relapsing disease and no curative options (n=10)
- Cohort 2: Batiraxcept plus cabozantinib 60 mg daily in patients with at least 1 prior therapy (n=25)
- Cohort 3: Batiraxcept plus cabozantinib 40 mg daily and nivolumab 240 q2w or 480 mg q4w in the 1st line setting (n=11)
The primary endpoint was investigator-assessed objective response rate by RECIST v1.1 criteria. The key secondary endpoint were safety, progression-free survival (PFS), and overall survival (OS).
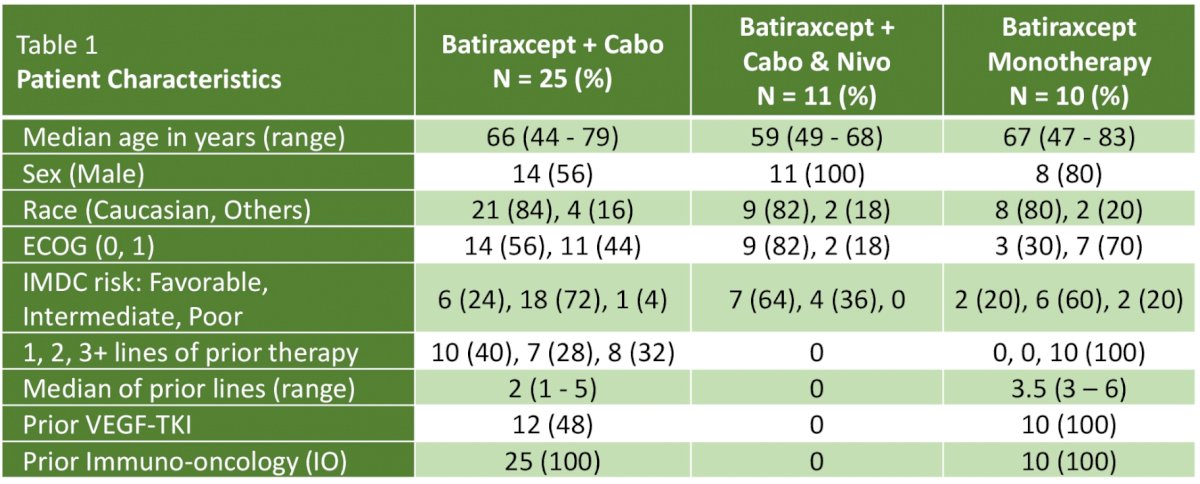
Enrolment has completed as of January 17, 2023. IMDC intermediate/poor risk patients accounted for 80%, 76%, and 27% of patients in cohorts 1, 2, and 3, respectively. The median prior lines of therapy were 4 and 2 in Cohorts 1 and 2, respectively. 100% and 88% of patients in Cohorts 1 and 2 had received prior immunotherapy, respectively, with 100% and 40% having specifically received VEGF-TKIs.
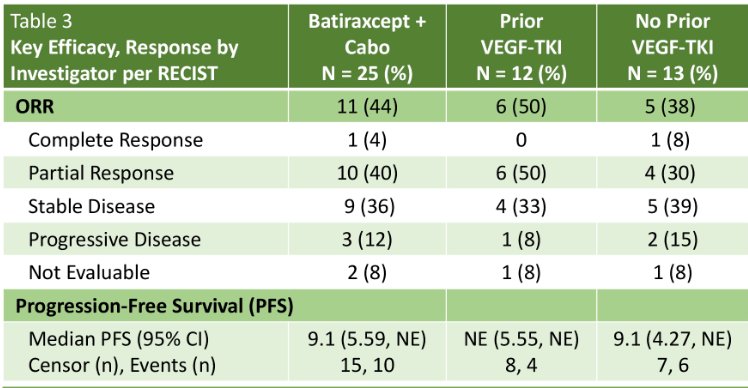
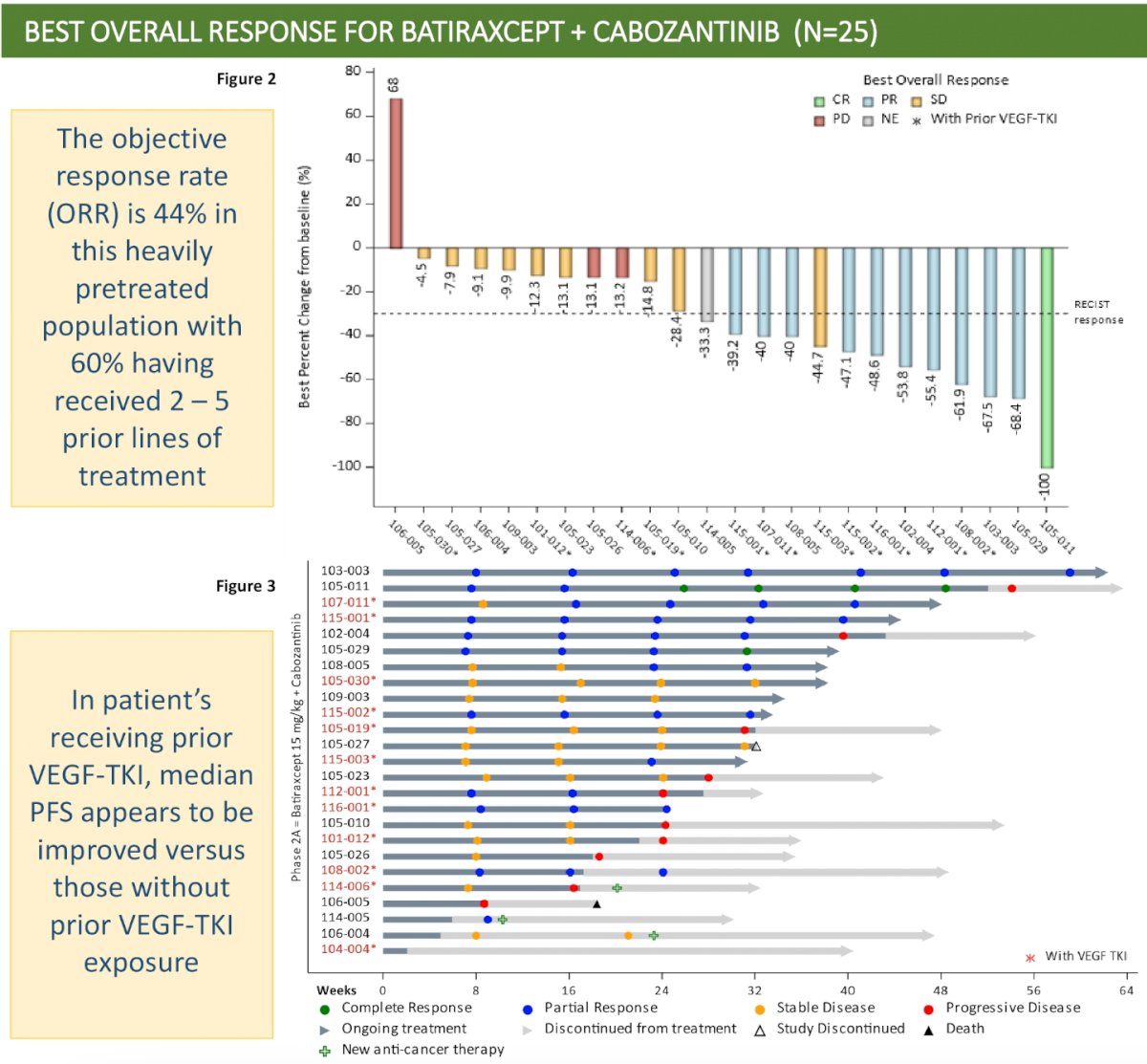
An ORR, defined as either a complete or partial response, was observed in none of the patients in Cohort 1, compared to 36% and 55% of patients in Cohorts 2 and 3, respectively. One patient (10%) in Cohort 1 had stable disease. The median PFS was 1.8, 7.2, and 7.6 months, respectively.
Grade ≥ 3 batiraxcept related adverse events were observed in 10%, 28%, and 46% of patients, respectively.

Dr. Beckermann concluded that batiraxcept monotherapy is well-tolerated but has limited clinical activity in heavily pre-treated patients with relapsing disease and no other curative options. Batiraxcept-based combinations demonstrated efficacy and tolerability in both treatment-naïve patients and those with at least 1 prior therapy. Given the encouraging safety and efficacy signals, batiraxcept + cabozantinib will be further studied in a phase 3 trial of ccRCC patients in the ≥2nd line setting, whose disease has progressed on prior immunotherapy and VEGF-TKI treatment. Exploratory analysis for a baseline serum soluble AXL/GAS6 ratio biomarker was found to predict clinical activity in the phase 1b study evaluating batiraxcept + cabozantinib in patients who had failed 1st line therapies; a similar analysis using this biomarker is ongoing for this patient population.
Presented by: Katy Beckermann, MD, PhD, Assistant Professor, Department of Medicine, Division of Hematology Oncology, Vanderbilt University Medical Center, Nashville, TN
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
Reference:- Shah NJ, et al. A phase 1b/2 study of batiraxcept (AVB-S6-500) in combination with cabozantinib in patients with advanced or metastatic clear cell renal cell carcinoma (ccRCC). J Clin Oncol 2023;41(6):Suppl.


