(UroToday.com) The 2022 ASCO annual meeting featured a session on prostate cancer, including a presentation by Dr. Maha Hussain discussing BRCAAway, a randomized phase 2 trial of abiraterone, olaparib, or abiraterone + olaparib in patients with mCRPC with DNA repair defects. The PARP-inhibitor olaparib is approved for mCRPC patients with deleterious germline or somatic homologous recombination repair gene mutations. PARP1 interacts with androgen signaling, and castration-resistant tumor cells exhibit increased PARP1 activity. Preclinically, PARP1-inhibition synergizes with androgen receptor targeted therapy. BRCAAway is a biomarker selected, randomized, open-label, multicenter phase 2 trial evaluating efficacy of targeting androgen receptor vs PARP vs combination in first line mCRPC patients with germline and/or somatic homologous recombination repair gene mutations in BRCA1, BRCA2, or ATM.
Eligible mCRPC patients underwent tumor next generation sequencing and germline testing. Patients with inactivating BRCA1, BRCA2 and/or ATM alterations were randomized 1:1:1 to Arm 1 abiraterone (1000 mg daily) + prednisone (5mg bid) (abiraterone + prednisone), Arm 2 olaparib (300 mg bid) or Arm 3 olaparib + abiraterone + prednisone. The primary endpoint is progression-free survival (PFS) analyzed using Kaplan-Meier estimates and Cox regression. Secondary endpoints include measurable disease response rate by RECIST, PSA-RR, undetectable PSA (≤ 0.2 ng/ml), and toxicity. Arms 1 and 2 patients were allowed to cross over at progression. Patients with other homologous recombination repair gene mutations were treated with olaparib; Arm 4 is ongoing and not presented at ASCO 2022. The trial schema for BRCAAway is as follows:
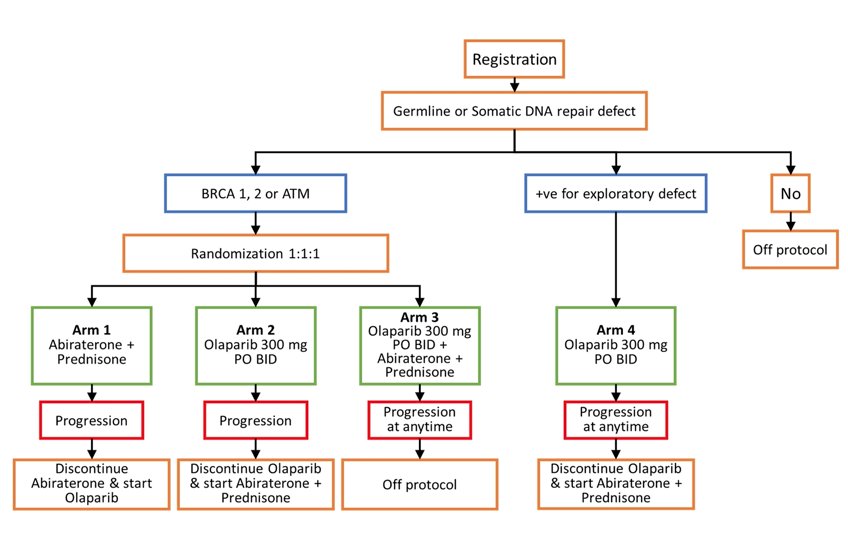
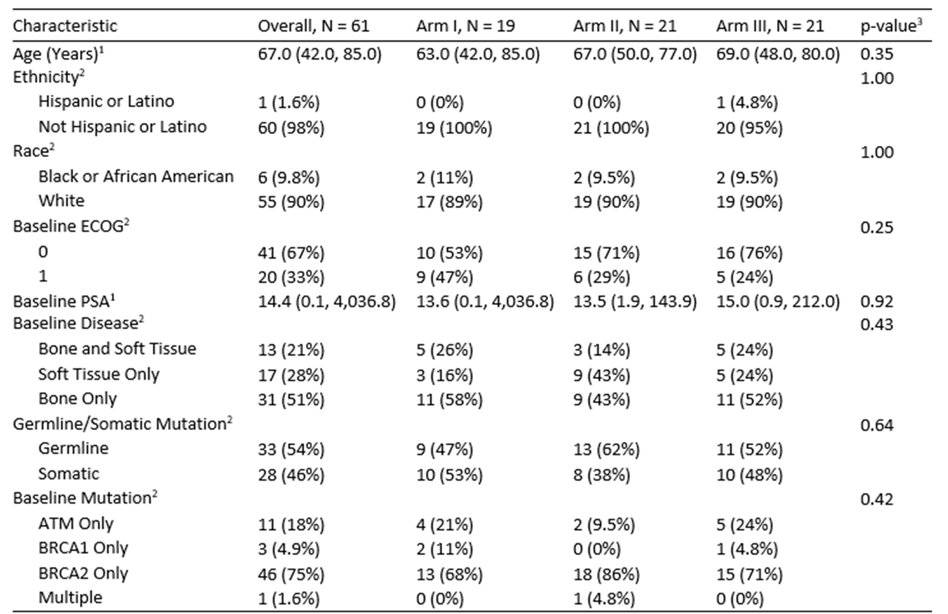
There were 161 patients registered and had next-generation sequencing, of which 61 patients were randomized to Arms 1-3. To date, 61 are evaluable for toxicity and 60 are evaluable for PFS. Baseline median age was 67 (range 42-85) years, 55 patients were White, 6 were Black, and the sites of disease are as follows: bone only (n=31), soft tissue only (n=17), bone and soft tissue (n=13). The median PSA was 14.4 ng/ml (range 0.1 – 4,036.8). Mutational status was: BRCA1 only n = 3, BRCA2 only n = 46, ATM only n = 11, and > 1 homologous recombination repair gene mutations n = 1. There were 33 patients (54%) that had germline and 28 (46%) had somatic mutations. The complete baseline characteristic is as follows:
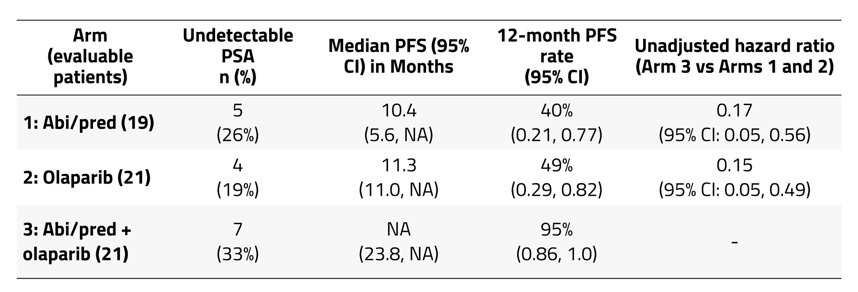
The median follow-up time was: 8.3 months (range 0.8, 33.3), 12.2 months (range 2.7, 21.8), and 16.8 months (range 2.9, 41.7) in Arms 1, 2, and 3, respectively. The median number of cycles was 8 (range 1, 43) for Arm 1, 11 (range 3, 31) for Arm 2, and 18 (range 3, 52) for Arm 3. There were 43 patients that had treatment-related adverse events, most commonly fatigue (23 patients; 1 grade 3, 22 grade 1/2), nausea (17 patients, grade 1/2), and anemia (9 patients, 2 grade 3, 7 grade 1/2). ≥50% PSA decline was reported in 79% of patients in Arm 1, 81% in Arm 2, and 90% in Arm 3. Median PSA nadir for Arms 1 was 2.17 ng/mL (95% CI 0.40, 49.27), for Arm 2 was 3.10 ng/mL (95% CI 0.83, 5.00), and for Arm 3 was 0.60 ng/mL (95% CI 0.10, 1.97), respectively. Undetectable PSA, median PFS, and 12-month PFS by Arm are listed as follows:
The Kaplan Meier curves for time to first progression by study arm is as follows:
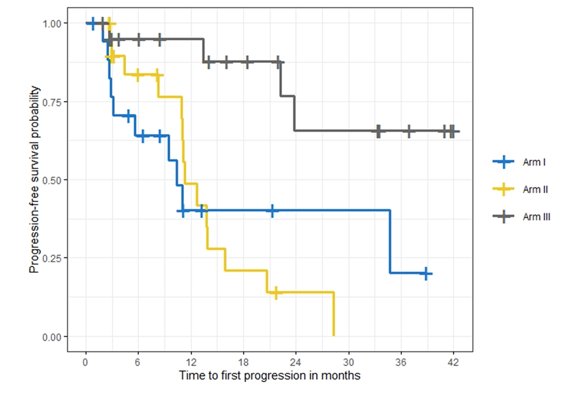
Dr. Hussain concluded her presentation discussing the randomized phase 2 BRCAAway by emphasizing that in mCRPC patients with inactivating BRCA1, BRCA2, and/or ATM alterations, abiraterone + prednisone + olaparib was well tolerated and resulted in longer PFS and better PSA response versus either agent alone.
Presented by: Maha H. A. Hussain, MD, FACP, FASCO, Robert H. Lurie Comprehensive Cancer Center, Northwestern University, Chicago, IL
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 3 – Mon, June 7, 2022.


