(UroToday.com) The 2022 ASCO annual meeting featured a session on prostate cancer, including a presentation by Dr. Andrew Armstrong discussing a post hoc analysis of ARCHES assessing radiographic progression in the absence of PSA progression in patients with mHSPC. In the phase III ARCHES trial, enzalutamide + ADT significantly reduced the risk of radiographic progression and increased overall survival in men with mHSPC, regardless of baseline PSA levels.1 This post hoc analysis offers a unique opportunity to evaluate if progression from mHSPC to mCRPC occurs without a rise in PSA in patients receiving enzalutamide + ADT versus ADT alone, as these findings may impact when routine imaging should be performed in clinical practice for mHSPC.
Patients with mHSPC (n = 1,150) were randomized 1:1 to enzalutamide (160 mg/day) + ADT or placebo + ADT. The concordance between radiographic progression and PSA progression, as defined by Prostate Cancer Clinical Trials Working Group 2 (PCWG2) criteria, and between any rise in PSA above nadir was assessed. Radiographic imaging with CT or MRI of the abdomen and pelvis, chest X-ray or CT, and bone scan were routinely performed at screening, week 13, and every subsequent 12 weeks. PSA levels were measured at screening, weeks 1, 5, and 13, every subsequent 12 weeks, and 30 days after the last dose of study drug or prior to initiation of new antineoplastic therapy, whichever occurred first.
In total, 267 of 1150 patients in ARCHES had radiographic progression (enzalutamide + ADT, n = 79; placebo + ADT, n = 188). At radiographic progression, the median PSA for enzalutamide + ADT-treated patients was 2.25 ng/mL (range: 0–1062.3 ng/mL) and 17.47 ng/mL (range: 0–1779.5 ng/mL) for placebo + ADT-treated patients. Most patients (67%) treated with enzalutamide + ADT did not have PCWG2-defined PSA progression at radiographic progression, compared with 57% of those treated with placebo + ADT:
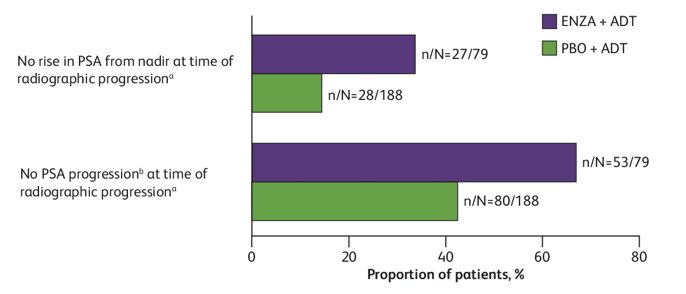
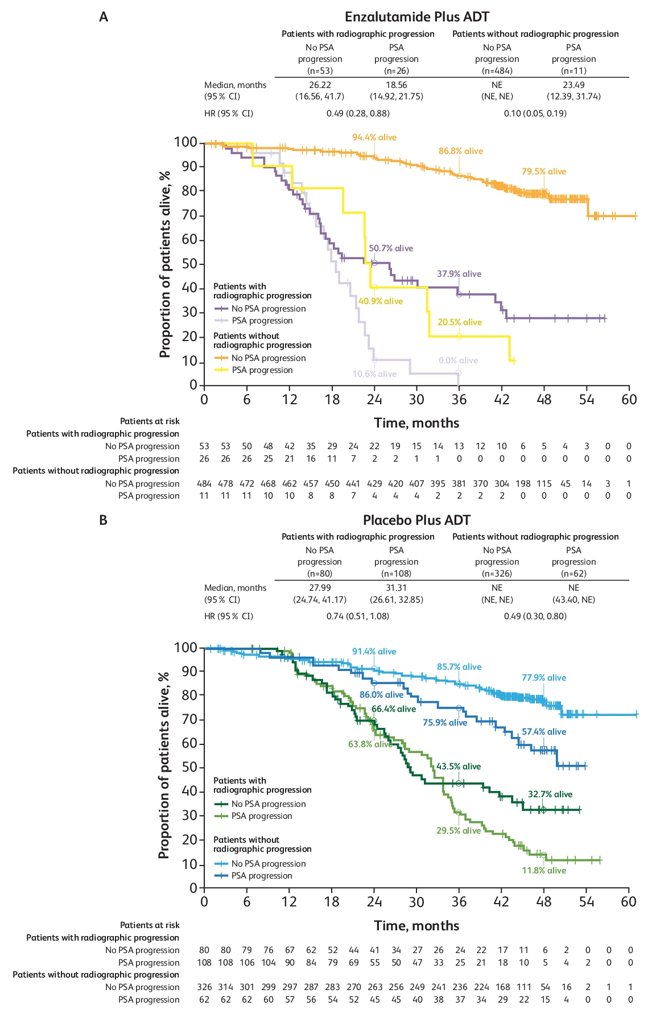
The median absolute and percentage rise in PSA from nadir to radiographic progression was 0.77 ng/mL and 200%, respectively, with enzalutamide + ADT compared with 12.23 ng/mL and 367%, respectively, with placebo + ADT. Patients who had radiographic progression had poorer survival outcomes in both treatment arms compared with those who did not have radiographic progression:
Dr. Armstrong concluded his presentation discussing a post hoc analysis of ARCHES assessing radiographic progression in the absence of PSA progression in patients with mHSPC with the following take-home messages:
- In this post hoc analysis of ARCHES, there was frequent discordance between radiographic progression and PSA progression by PCWG2 criteria or any PSA rise over nadir in patients with mHSPC treated with enzalutamide + ADT
- Survival outcomes remain worse for patients with radiographic progression (with or without PSA progression) compared with outcomes of non-progressors
- Regular imaging is recommended to detect radiographic progression among patients treated with potent androgen receptor pathway inhibitors, such as enzalutamide + ADT, as serial PSA monitoring alone may not be sufficient to detect radiographic progression in many patients
Presented by: Andrew J. Armstrong, MD, MSc, Duke Cancer Institute Center for Prostate and Urologic Cancers, Duke University School of Medicine, Durham, NC
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 3 – Mon, June 7, 2022.
References:


