(UroToday.com) The 2022 ASCO annual meeting featured a session on prostate cancer, including a presentation by Dr. Eugene Shenderov discussing a phase 2 trial in localized prostate cancer using the anti-B7-H3 antibody enoblituzumab. B7-H3/CD276, a member of the B7 superfamily, is highly expressed in prostate cancer and is associated with rapid biochemical recurrence and early metastases:
B7-H3 is the only checkpoint candidate to have a presumptive androgen receptor binding site, suggesting interaction with the androgen axis. Enoblituzumab (MacroGenics) is an investigational humanized Fc-optimized B7-H3–targeting antibody that induces antibody dependent cellular cytotoxicity. The hypothesis of this study was that neoadjuvant enoblituzumab treatment in patients with high-risk localized prostate cancer will lead to reduced biochemical recurrence following prostatectomy, by modulating T cell immunity in the tumor microenvironment and also direct tumor killing via ADCC.
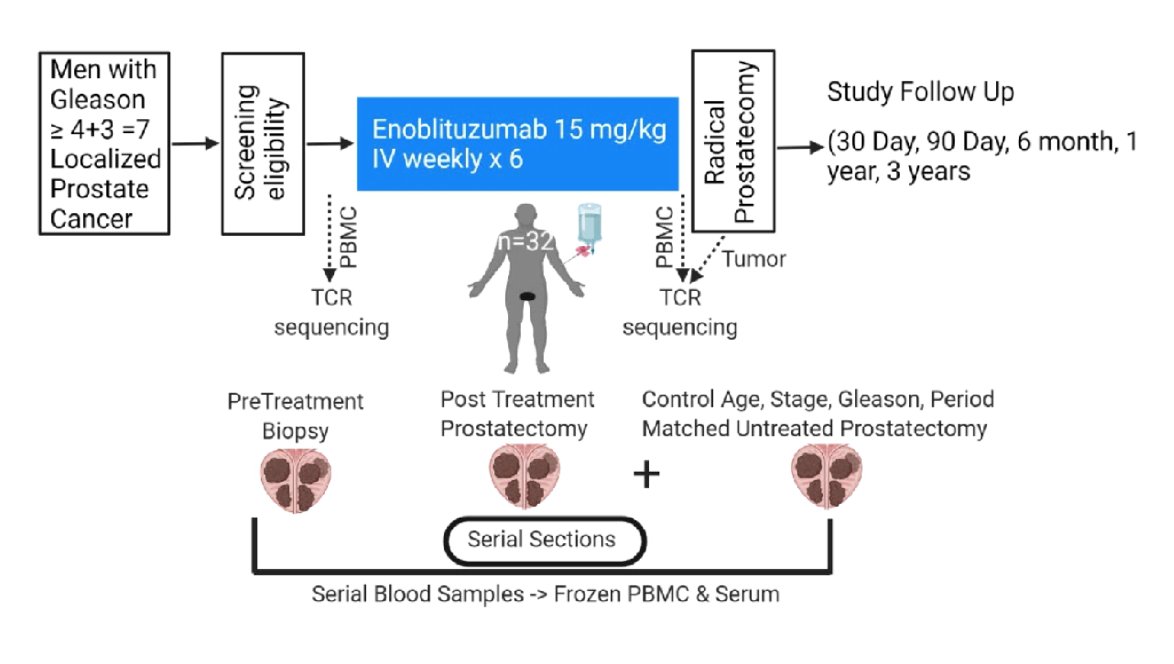
In this phase 2 single-arm biomarker-rich neoadjuvant trial, men with operable intermediate- and high-risk localized prostate cancer (Grade Groups 3-5) were enrolled to evaluate the safety, anti-tumor efficacy, and immunogenicity of enoblituzumab when given prior to prostatectomy. Patients received enoblituzumab (15 mg/kg IV weekly x 6) prior to surgery. Prostate glands were removed 2 weeks after the last dose, and were examined for pathologic and immunologic endpoints. The co-primary outcomes were safety and PSA0 at 1 year post-op. Pre-planned secondary outcomes were PSA and Gleason grade group change from biopsy to prostatectomy. The trial design for this study is as follows:
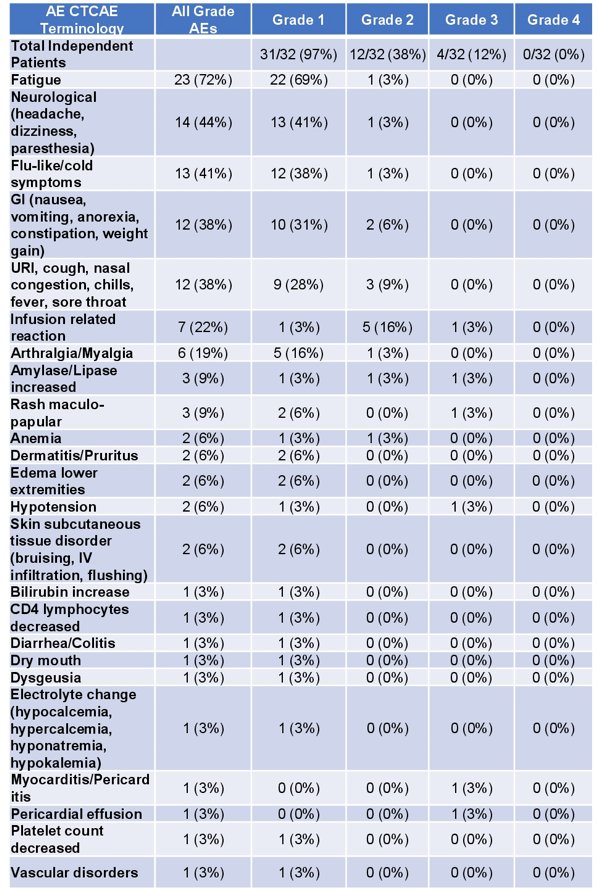
There were 32 men enrolled in this clinical trial, and grade 3/4 adverse events occurred in 12% of patients. One patient developed a grade-3 infusion reaction, and one had immune myocarditis that improved with steroids. A full list of the treatment-related adverse events by type and grade are as follows:
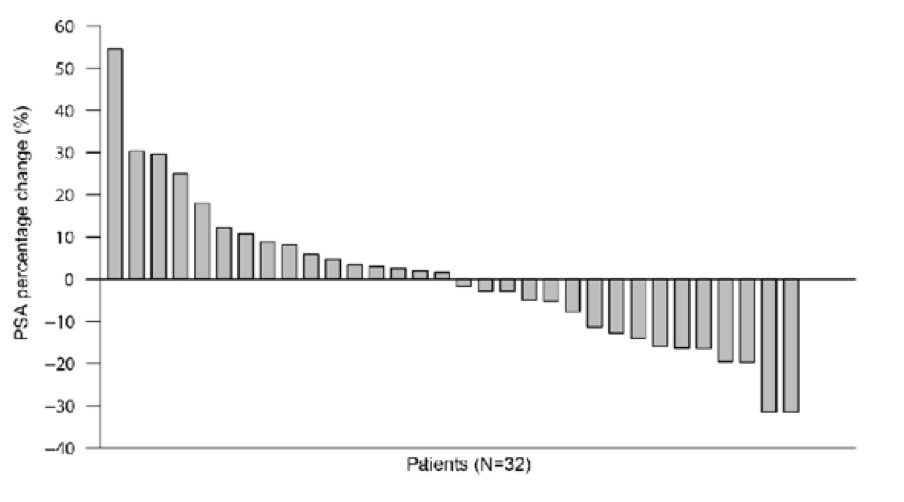
Pre-prostatectomy PSA declines of >10% were observed in 31% of patients (95% CI 16-50%):
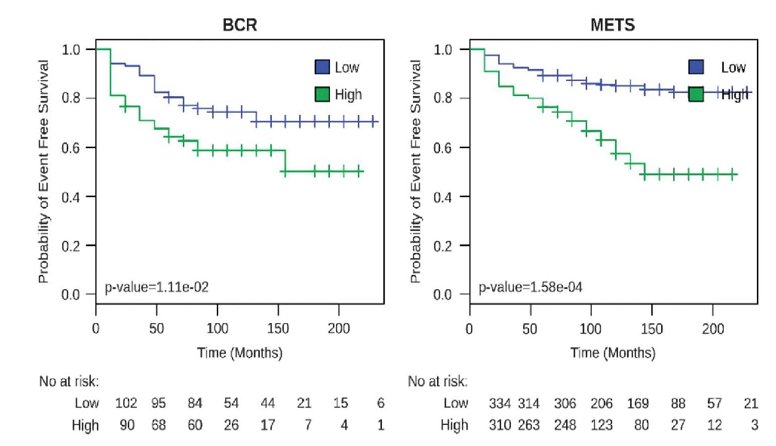
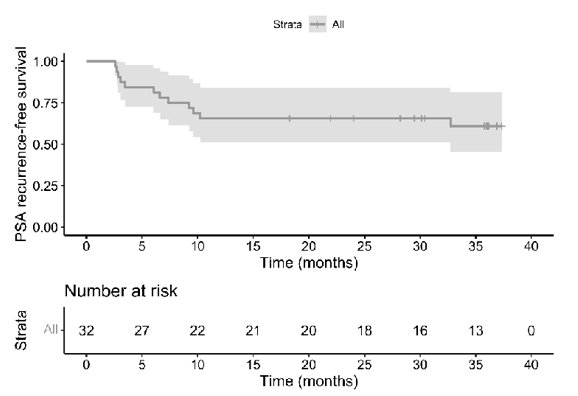
PSA0 at 1 year post-op was seen in 66% of men (95% CI 47-81%). The median time to PSA recurrence was not reached, with a median follow-up of 30 months:
Gleason group upgrade was observed in 13% of patients, no change in 37%, and downgrade in 50% of patients. Gleason grade group changes were significantly associated with enoblituzumab treatment compared to 1:1 matched historical controls (p=0.023). Tumor microenvironment profiling by NanoString GeoMx spatial proteomics and PanCancer IO 360 mRNA expression analysis demonstrated post-treatment upregulation of CD8+ T cells, PD-1/PD-L1 expression, and immune activation (granzyme B, IFN signaling, myeloid inflammation). Additionally, there was a significant association between CD8+ T-cell increases and Gleason grade group declines. First-in-human antigen spread profiling revealed no safety concerns. Finally, TCR sequencing showed focused peripheral expansion of tumor associated T-cell clones that correlated with PSA0 at 1 year.
Dr. Eugene Shenderov concluded his presentation discussing a phase 2 trial in localized prostate cancer using the anti-B7-H3 antibody enoblituzumab with the following take-home messages:
- In this neoadjuvant trial, inhibition of B7-H3 with 6 weeks of enoblituzumab demonstrated favorable safety and encouraging activity in localized prostate cancer patients prior to prostatectomy
- Data suggest robust intratumoral induction (adaptive upregulation) of immune checkpoints, T-cell activation, and myeloid inflammation
- Enoblituzumab-induced peripheral expansion of tumor associated T-cell clones may be associated with tumor control
Presented by: Eugene Shenderov, MD, PhD, Johns Hopkins University School of Medicine, Baltimore, MD
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 3 – Mon, June 7, 2022.


