(UroToday.com) The 2022 ASCO annual meeting featured a session on prostate cancer, including a presentation by Dr. Antoine Thiery-Vuillemin discussing further results from the PROpel trial assessing tolerability of abiraterone combined with olaparib in patients with mCRPC. At the first data cut-off for the primary endpoint of investigator-assessed radiographic progression free survival (rPFS), the Phase III PROpel (NCT03732820) trial demonstrated a statistically significant CRP clinical benefit from combining olaparib + abiraterone in the first-line mC setting vs placebo + abiraterone. Benefit was seen irrespective of a patient’s homologous recombination repair mutation status, with a median rPFS of 24.8 months for olaparib + abiraterone vs 16.6 months for placebo + abiraterone (HR 0.66, 95% CI 0.54–0.81; p < 0.0001). The safety profile of olaparib + abiraterone was shown to be consistent with that for the individual drugs. At the 2022 ASCO meeting, Dr. Thiery-Vuillemin and colleagues reported additional interim safety analysis from PROpel.
Eligible patients were ≥18 years with mCRPC, had received no prior chemotherapy or next-generation hormonal agent treatment at mCRPC stage, and were unselected by homologous recombination repair mutation status. Patients were randomized 1:1 to abiraterone (1000 mg daily) plus prednisone/prednisolone with either olaparib (300 mg bid) or placebo. The primary endpoint was investigator-assessed rPFS, and safety was assessed in all patients receiving ≥1 dose of study treatment by adverse event reporting (CTCAE v4.03).
There were 398 patients that received olaparib + abiraterone and 396 received placebo + abiraterone (safety analysis set). At the time of data cut-off (July 30, 2021), the median total duration of exposure for olaparib was 17.5 vs 15.7 months for placebo, and for abiraterone 18.2 months in the olaparib + abiraterone arm and 15.7 in the placebo + abiraterone arm. For those receiving olaparib, the median time to onset of adverse events was within the first 4 months, recovering within the first 6 months (except fatigue):
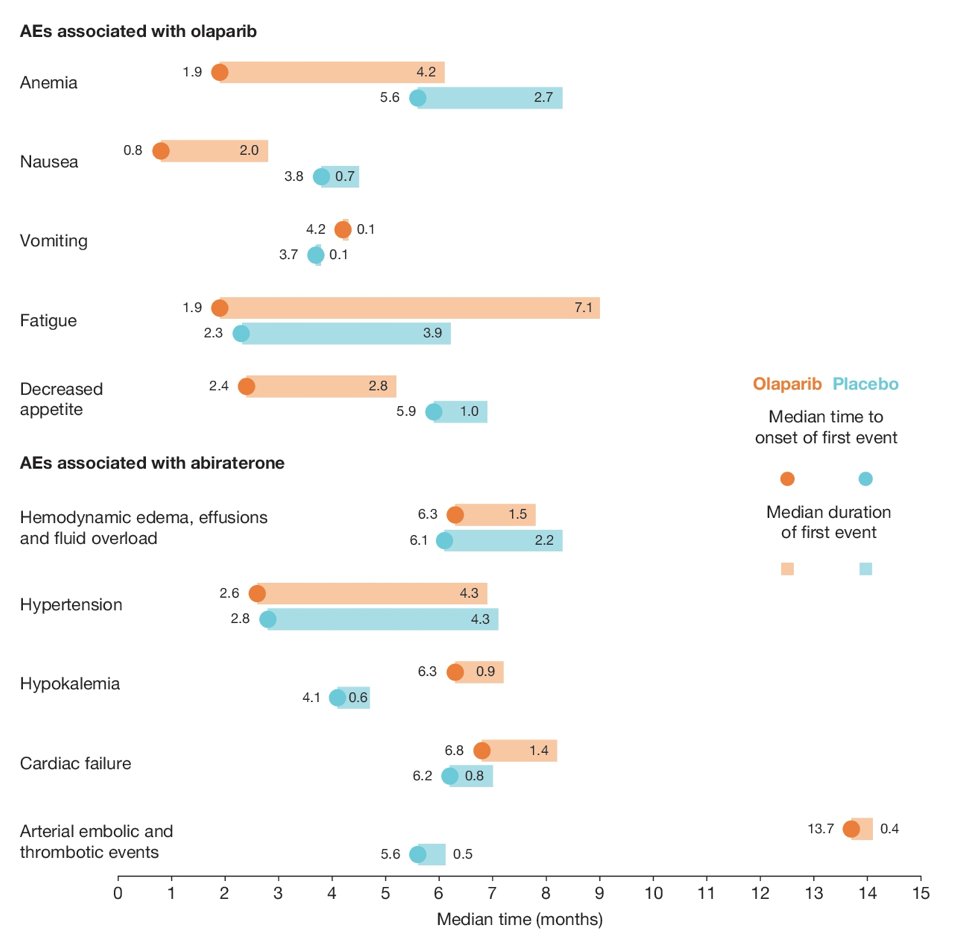
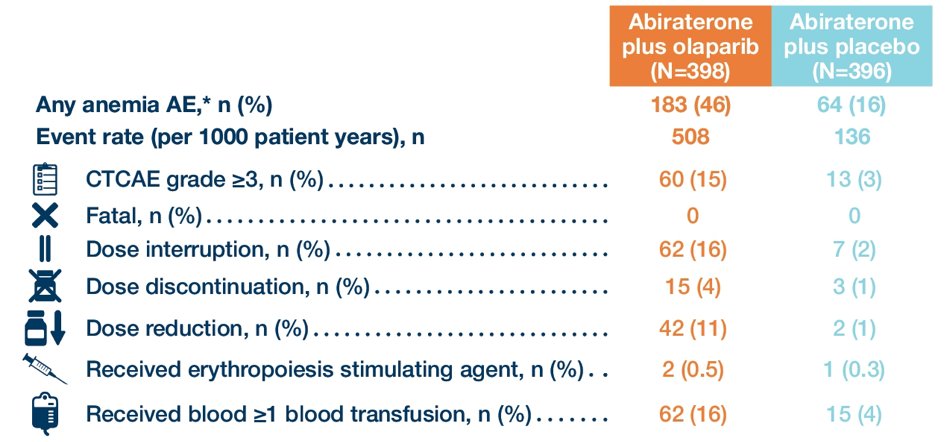
Anemia (n=183) was the most common adverse event in the olaparib + abiraterone arm, and 34% of these 183 events were managed by dose interruption, 23% by dose reduction, and 8% resulted in treatment discontinuation:
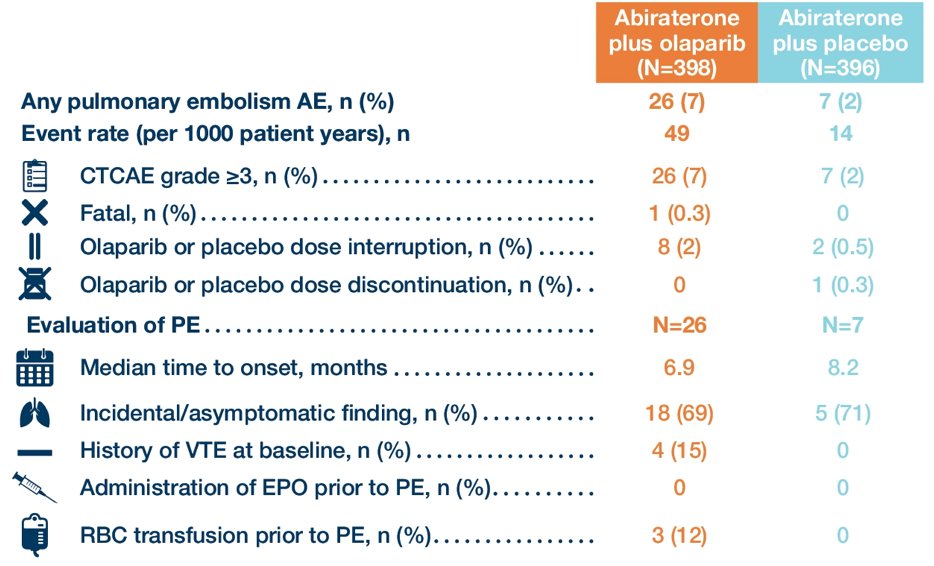
Anemia and pulmonary embolism were the only Grade ≥3 adverse events in ≥5% of patients (anemia: olaparib + abiraterone, 15.1% vs placebo + abiraterone, 3.3%; pulmonary embolism: 6.5% vs 1.8%, respectively). Most pulmonary embolisms were detected incidentally on radiographic imaging (69.2% and 71.4% in the olaparib + abiraterone and placebo + abiraterone arms, respectively) and no patients discontinued treatment:
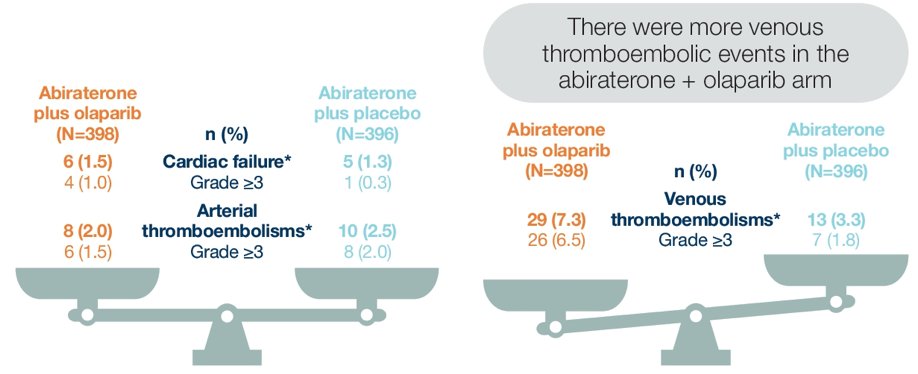
More patients in the olaparib + abiraterone arm experienced venous thromboembolism, and arterial thromboembolism and cardiac failure adverse events were balanced between the treatment arms:
No adverse event of myelodysplastic syndrome/acute myeloid leukemia was reported in either treatment arm. COVID-19 was reported more frequently with olaparib + abiraterone (8.3% vs 4.5%).
Dr. Thiery-Vuillemin concluded this presentation by discussing further results from the PROpel trial assessing tolerability of abiraterone combined with olaparib in patients with mCRPC with the following take-home messages:
- PROpel demonstrated a predictable safety profile for olaparib + abiraterone given in combination to patients with first-line mCRPC unselected by homologous recombination repair mutation status
- Anemia was the most common adverse event occurring with abiraterone and olaparib treatment, and the majority of anemia adverse events were managed with olaparib dose reductions or temporary interruptions
- Adverse events of cardiac failure and arterial thromboembolism were reported at similar frequency in both treatment arms
- Pulmonary embolism events occurred more frequently in the abiraterone + olaparib arm, with the majority of these events being asymptomatic and none leading to treatment discontinuation in the abiraterone + olaparib arm
- The safety profile of abiraterone was not adversely impacted by its combination with olaparib
- The overall results of PROpel demonstrate the clinical benefits of olaparib in combination with abiraterone in the first-line treatment of an unselected population of patients with mCRPC
Presented by: Antoine Thiery-Vuillemin, CHRU Besançon Hôpital J.Minjoz, Besançon, France
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 3 – Mon, June 7, 2022.


