(UroToday.com) The 2022 ASCO annual meeting featured a session on prostate cancer, including a presentation by Dr. Wesley Armstrong discussing results from the prospective randomized phase 3 trial PSMA SRT assessing the impact of PSMA PET/CT on prostate cancer salvage radiotherapy management. In high-risk patients, biochemical recurrence after salvage radiotherapy reaches nearly 50%. Despite improved post-salvage radiotherapy control from RTOG consensus fields and risk-management recommendations, relapse rates remain as high as 50% at 5 years for high-risk patients. PSMA PET/CT has been shown to detect recurrent disease beyond consensus radiation fields at low PSA threshold. Integration of recently FDA approved PSMA PET tracers into salvage radiotherapy planning may improve biochemical recurrence-free survival through management adapted around previously undetectable gross tumor volumes. The purpose of the randomized PSMA SRT trial is to compare the success rate of salvage radiation therapy for recurrence of prostate cancer after radical prostatectomy with (intervention arm) and without (control arm) planning based on PSMA PET/CT. At ASCO 2022, Dr. Armstrong and colleagues reported the secondary endpoint of the trial: impact of PSMA PET/CT on the treatment plan.
This is a randomized, controlled, prospective, open label, phase 3 clinical trial with institutional funding. There were 193 patients randomized to proceed with standard salvage radiation therapy with any conventional imaging aside from PSMA PET/CT (control arm) or undergo a 68Ga-PSMA-11 PET/CT scan prior to salvage radiation therapy (investigational arm). The following information was collected on case-report forms before randomization (intended salvage radiation therapy plan) and after treatment:
- Radiation field region (prostate fossa, pelvic lymph node)
- Total dose
- Dose per fraction
- Duration
- ADT use and duration
- PSMA influence on target volume
- Other (free-text)
Changes between salvage radiation therapy plan before randomization and delivered treatment were classified as Major, Minor or No Change. Major change: change of ADT duration ≥3 months, change of standard radiotherapy volumes (prostate fossa and pelvic lymph node), target volume delineation beyond standard radiotherapy field, simultaneous-integrated boost beyond standard radiotherapy fields, and initiation of advanced systemic therapy (novel ADT agents, chemotherapy). Minor change: simultaneous-integrated boost within standard radiotherapy fields. Fisher exact test was used to compare prevalence of events between study arms.
There were 193 patients enrolled from September 6, 2018, to August 17, 2020: 90 randomized to the control group and 103 randomized to the PSMA group:
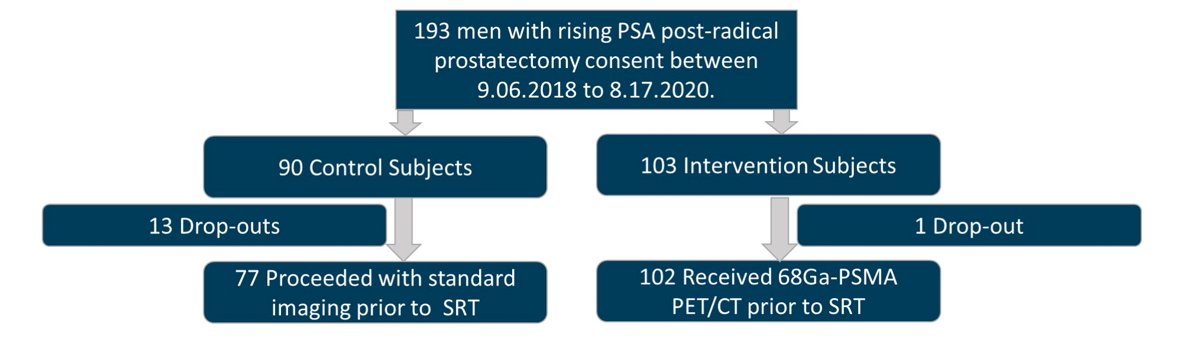
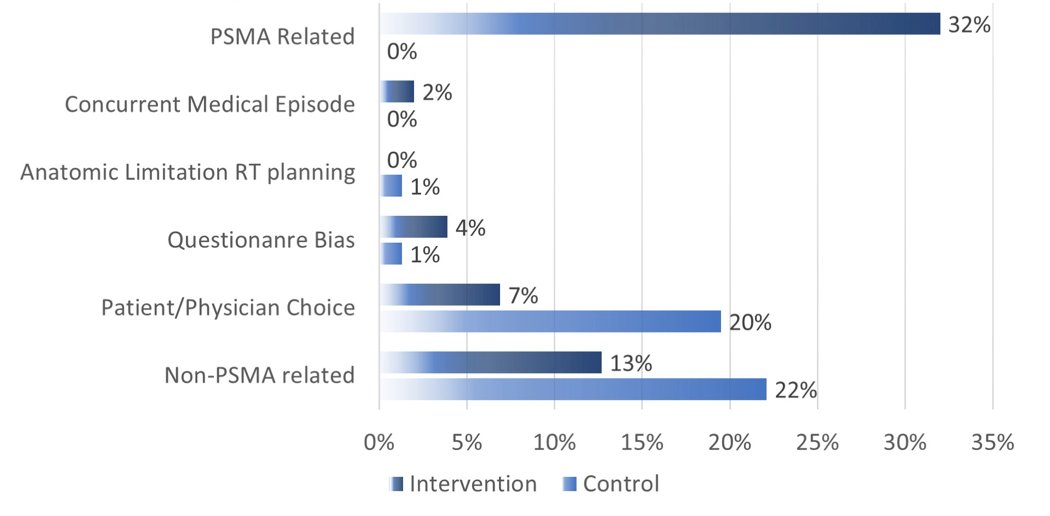
The median time from radical prostatectomy to enrollment and median PSA was 20.3 months (IQR 1.4–245) and 0.3 ng/ml (IQR 0.2-10.3) for the control group, and 28.3 months (IQR 1.2–21) and 0.23 ng/ml (IQR 0.1-29.9) for the PSMA group. The control arm had 13 dropouts (17%) while the intervention had one (1%). PSMA was positive in 38/102 (38%): 12/102 (12%) outside of pelvis, and 20/102 (20%) in pelvic lymph nodes. Pre-randomization radiotherapy plan and delivered radiotherapy plan were available in 193/193 (100%), (77/90 control (86%) and 102/103 PSMA (99%), (p = 0.0004), respectively. There were 0/77 (0%) and 7/102 (7%) minor changes in the control and PSMA groups (p = 0.02). There were 17/77 (22%) and 46/102 (45%), major changes (p = 0.004), with 33/45 (73%) being PSMA-related. A summary of the change in management is as follows:
Treatment escalation occurred in 7/17 (41%) and 36/52 (69%) (p = 0.048), and de-escalation in 10/17 (59%) and 10/52 (19%) (p = 0.004). Nine/102 patients (9%) received advanced systemic therapy in relation to PSMA findings whereas only 1/77 (1%) patient in the control received advanced therapy (p = 0.044). The primary endpoint analysis, patients who received salvage radiotherapy without evidence of extra-pelvic metastatic disease, will be monitored to attain impact on 5-year biochemical recurrence-free survival.
Dr. Armstrong concluded this presentation discussing results from the prospective randomized phase 3 trial PSMA SRT assessing the impact of PSMA PET/CT on prostate cancer salvage radiotherapy management noting that PSMA PET findings provided information which initiated major management changes to salvage radiotherapy planning in 32% of patients.
Presented by: Wesley R. Armstrong, Ahmanson Translational Theranostics Division, University of California, Los Angeles, CA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 3 – Mon, June 7, 2022.


