(UroToday.com) At the 2022 American Society of Clinical Oncology Annual Meeting held in Chicago and virtually, the poster session focused on Prostate, Testicular, and Penile cancers on Monday afternoon included a presentation from Dr. Michael A. Gorin examining subgroup analyses of the OSPREY trial of Piflufolastat F18-PET/CT.
Prostate-specific membrane antigen (PSMA) PET/CT based imaging is gaining a rapidly increasing role in the management of men with prostate cancer including in initial staging of patients with high-risk disease, in biochemical recurrence, and in the assessment of patients for theranostic treatments. The OSPREY clinical trial was a phase 2/3 prospective study of using PSMA PET/CT with the tracer piflufolastat F18 (NCT02981368). Piflufolastat F18 (aka 18F-DCFPyL or PyL) is a novel PSMA-targeting radiopharmaceutical approved for imaging of prostate cancer patients both at initial staging and for disease recurrence. In the abstract, Dr. Gorin and colleagues described SUV results of Piflufolastat F18 imaging in the OSPREY trial stratified by biopsy status, baseline PSA levels, and Gleason score (GS).
In the OSPREY trial, men with NCCN high-risk prostate cancer were scheduled to undergo radical prostatectomy with pelvic lymphadenectomy (RP-PLND) (Cohort A) and men with radiologically suspected recurrent/metastatic prostate cancer (Cohort B) underwent Piflufolastat F 18-PET/CT.

Patients received single IV dose of 9 mCi (333 MBq) of piflufolastat F 18 followed by PET/CT acquisition 1-2 hours later. Piflufolastat F 18 uptake in various lesion locations as defined by maximum and peak SUV (SUVmax, SUVpeak) was determined by three blinded, independent central readers for each tissue (e.g., bone, lymph nodes (LN), soft tissue). To measure SUVs, the reader placed a volume of interest (VOI) on each identified lesion. SUVmax was defined as the maximum single-voxel SUV within the VOI. SUVpeak within the VOI was defined as the average SUV within a fixed-sized VOI (1 cm diameter sphere), representing the cluster with the highest average SUV.
In this trial, 345 men underwent piflufolastat F 18-PET/CT. Among men with radiologically suspected recurrent/metastatic prostate cancer (n=93) in cohort B, SUVmax and SUVpeak were significantly higher for biopsy positive lesions when compared to biopsy negative lesions from bone and LN. Further, SUVpeak for biopsied bone and LN appeared to increase with rising baseline PSA.
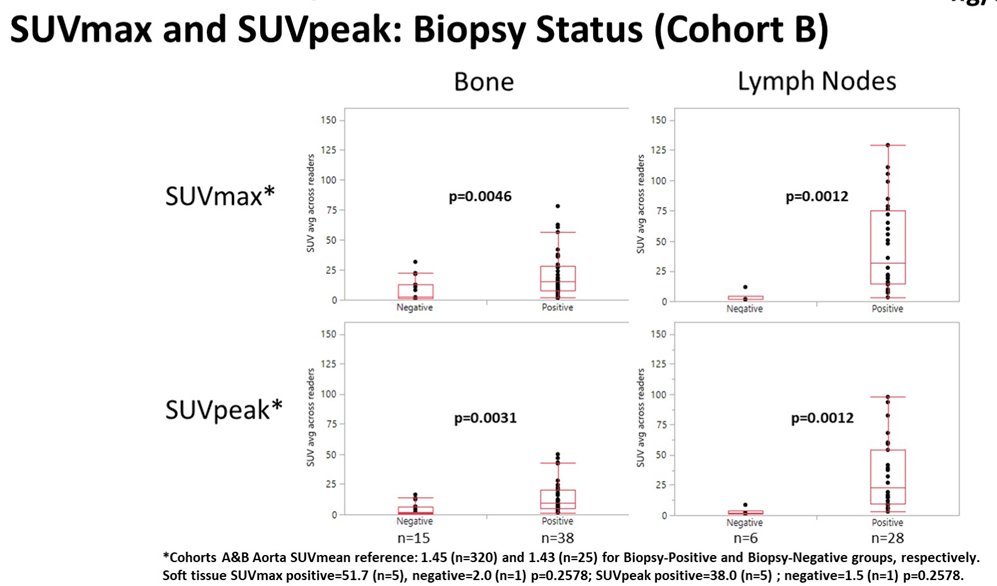
In men with NCCN high-risk prostate cancer scheduled to undergo radical prostatectomy with pelvic lymphadenectomy (RP-PLND) (n=252) in Cohort A, SUVpeak within the prostate increased with baseline PSA and was highest for those men with GS 9-10 histology.
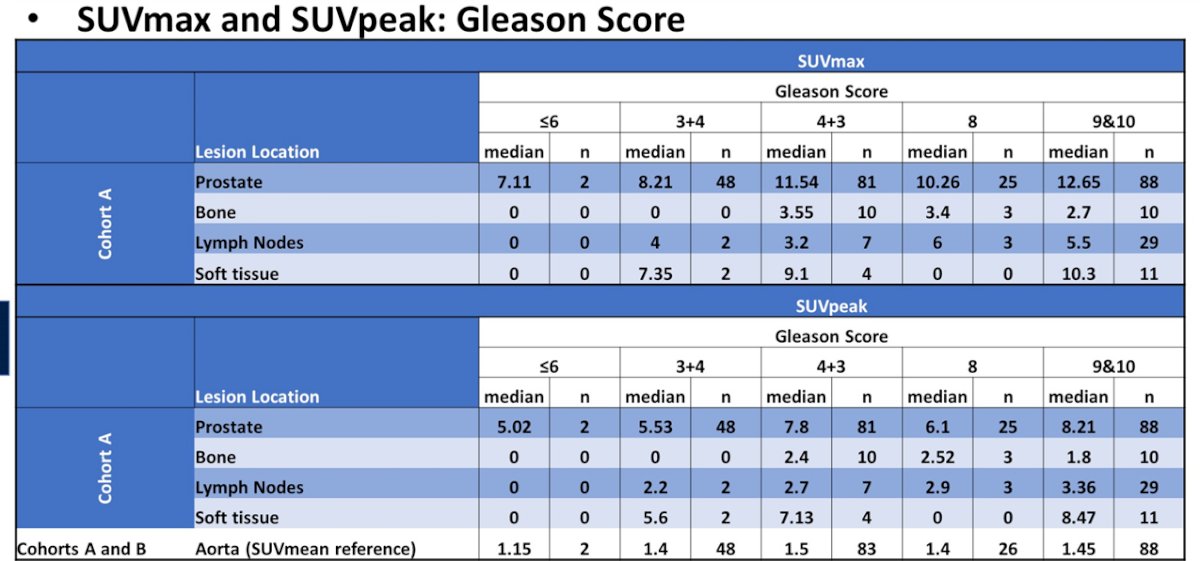
Thus, the authors conclude that Piflufolastat F 18-PET/CT uptake was significantly higher in biopsy positive lesions and increased with baseline PSA. Prostate SUVpeak was highest for men with GS 9-10 histology.
Presented by: Michael Gorin, MD, Clinical Assistant Professor, Department of Urology, University of Pittsburgh School of Medicine, Pittsburgh, PA, Staff Urologist, UPMC Western Maryland, Cumberland, MD


