(UroToday.com) The 2022 ASCO annual meeting featured an oral abstract session on prostate cancer, including a discussant presentation by Dr. Russell Szmulewitz discussing making sense of the intensified mHSPC landscape. Dr. Szmulewitz notes that in a University of Chicago lab long ago, Dr. Charles Huggins noted in 1940 that testosterone removal lead to prostate shrinkages in dogs and that androgen removal was beneficial in patients with prostate cancer (1941). This led to him winning the Nobel Prize in Medicine and to note that “prostate cancer is influenced by androgenic activity in the body.” Dr. Szmulewitz notes that intensification means building on ADT, with the following figure highlighting clinical trials in mHSPC (also, STAMPEDE docetaxel, abiraterone, cytoreductive radiation; PEACE-1):
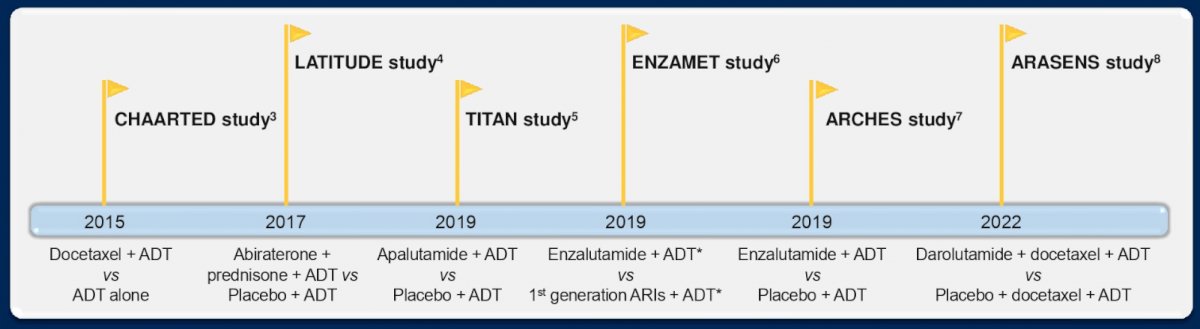
According to Dr. Szmulewitz, there are 5 key questions for the mHSPC field:
- Do all patients with mHSPC benefit from ADT intensification?
- When should we use triplet (ADT + ARSI + docetaxel therapy)?
- How do we choose the ARSI “intensifier”?
- Is there any role for molecularly targeted precision medicine?
- How can we accelerate innovation?
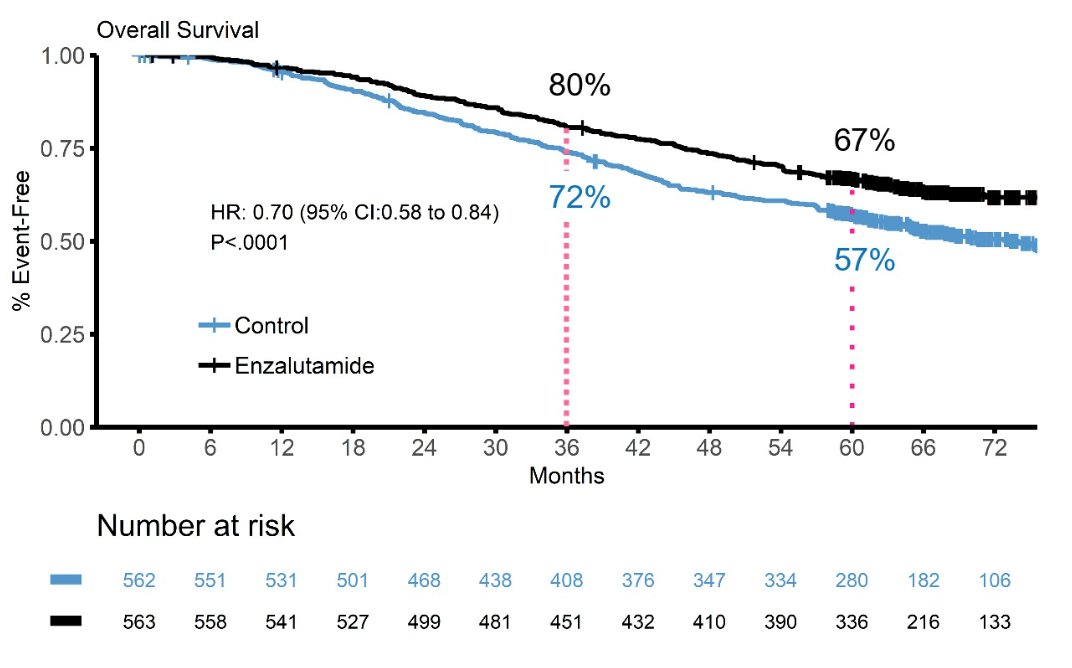
As part of this discussant presentation, Dr. Szmulewitz highlighted the following three abstracts “Updated overall survival outcomes in ENZAMET (ANZUP 1304), an international, cooperative group trial of enzalutamide in mHSPC” presented by Dr. Ian Davis, “A phase 3 trial of SHR3680 versus bicalutamide in combination with ADT in patients with high-volume mHSPC”, presented by Dr. Ding-Wei Ye, and “Assessing intermediate clinical endpoints as potential surrogates for overall survival in men with mHSPC” presented by Dr. Susan Halabi. Discussing the ENZAMET updated survival, the effect of prognostic grouping, and docetaxel abstract, Dr. Szmulewitz notes that prior to randomization, testosterone suppression up to 12 weeks and up to 2 cycles of docetaxel were allowed. Additionally, the control arm was a robust control arm with testosterone suppression + standard non-antiandrogen. Notably, the OS benefit of enzalutamide persists with longer follow-up. OS results in the combined cohort were based on 476 deaths, with a median OS in the enzalutamide of not reached (95% CI not reached – not reached) compared to the control group of 73.2 months (95% CI 64.7 – not reached). The 5-year overall survival in the enzalutamide arm was 67% compared to 57% in the control arm:
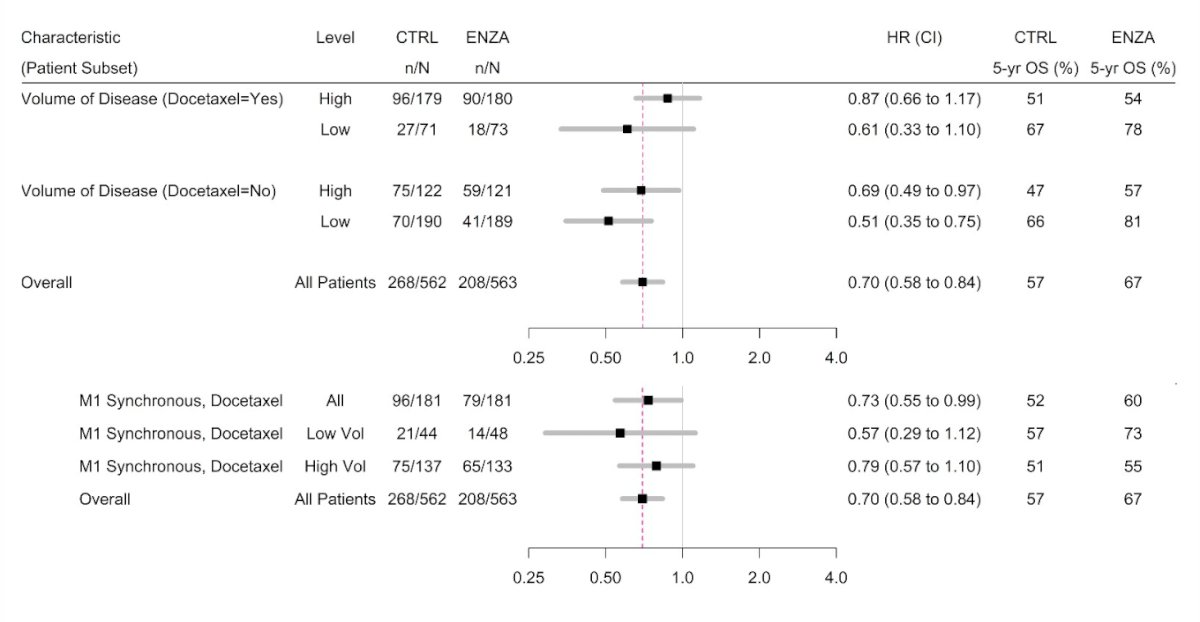
Additionally, there was a substantial crossover in the control arm to enzalutamide or abiraterone for mCRCP, with 76% of those in the control arm receiving enzalutamide or abiraterone after progression, compared to 26% in the enzalutamide arm. Dr. Szmulewitz also noted that the OS benefit of enzalutamide was apparent regardless of volume of disease or docetaxel use, with the most apparent benefit in low-volume, no-docetaxel (HR 0.51, 95% CI 0.35-0.75) patients. Additionally, there was a benefit in the de novo docetaxel population:
Dr. Szmulewitz’s take home messages from the ENZAMET presentation are as follows:
- Enzalutamide had superior OS versus non-steroidal antiandrogen, despite most patients in the control arm “crossing over”
- Longer follow-up has helped clarity the benefit for de novo disease + docetaxel
- The strengths of the study are that there was an active control, “real-world” population mix, with long follow-up
- The weaknesses of the study are that receipt of docetaxel was not randomized and was not powered for analyses
- Does this change clinical practice? Not really, as enzalutamide is already widely used in mHSPC, but there is perhaps more rationale for enzalutamide + docetaxel
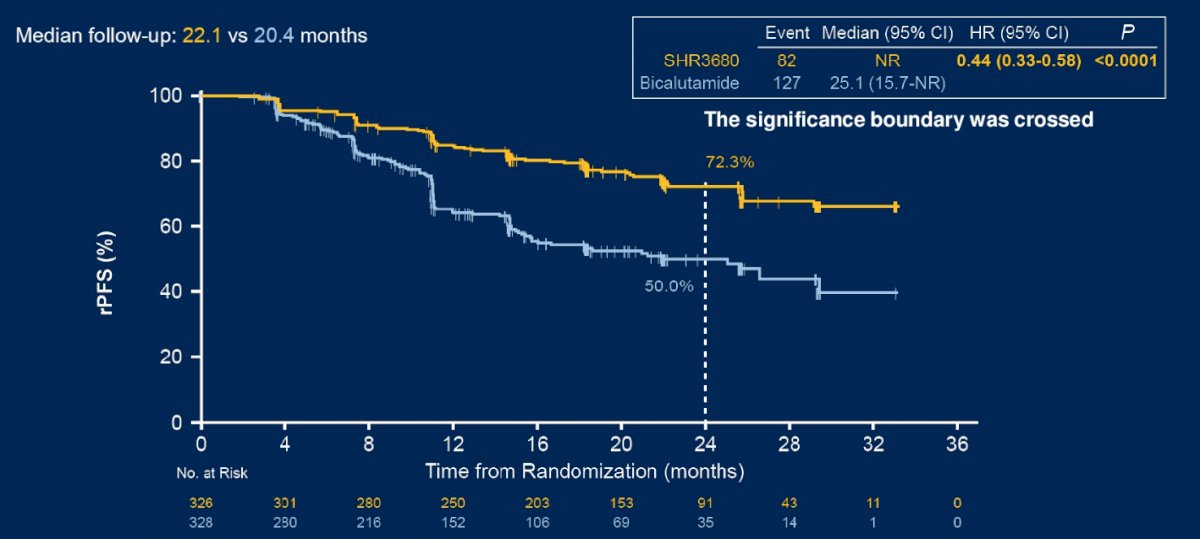
Dr. Szmulewitz then discussed the CHART trial, which was a phase 3 trial of SHR3680 versus bicalutamide in combination with ADT in patients with high-volume mHSPC. With regards to study design, Dr. Szmulewitz notes that 90% of patients were from China, 20% had visceral disease, and ~90% had de novo metastatic disease. SHR3680 significantly reduced the risk of radiographic progression or death than bicalutamide (HR 0.44, 95% CI 0.33-0.58; p < 0.0001; median, not reached vs 25.1 months):
OS data were immature but an improved OS was observed in SHR3680 group (median not reached) compared to bicalutamide group (median not reached; HR 0.58, 95% CI 0.42-0.80; p = 0.0009):

Dr. Szmulewitz highlighted that there were no outliers on subgroup analysis other than visceral disease, and secondary outcome hazard ratios all favored SHR3680:
- Investigator-assessed rPFS: HR 0.39, 95% CI 0.30-0.50
- Time to PSA progression: HR 0.21, 95% CI 0.16-0.27
- Time to next skeletal related event: HR 0.65, 95% CI 0.50-0.84
- Time to new anti-prostate cancer therapy: HR 0.33, 95% CI 0.26-0.41
Additionally, SHR3680 was well tolerated, with <1% of adverse events leading to discontinuation and ~6% of adverse events leading to dose interruption. Dr. Szmulewitz’s take home messages from the CHART trial are as follows:
- SHR3680 was superior to bicalutamide for mHSPC
- The strengths of the study are that there was an active control, with an efficient design
- The weaknesses of the study are that the results may not be applicable to a worldwide patient population
- Does this change clinical practice? No, more data is needed outside of China and there is question as to whether OS persists with longer follow-up
Dr. Szmulewitz then discussed the abstract assessing intermediate clinical endpoints for OS in men with mHSPC. Dr. Szmulewitz highlighted that rPFS/cPFS track along with OS, but happen earlier, and that PFS and OS correlate irrespective of arm:
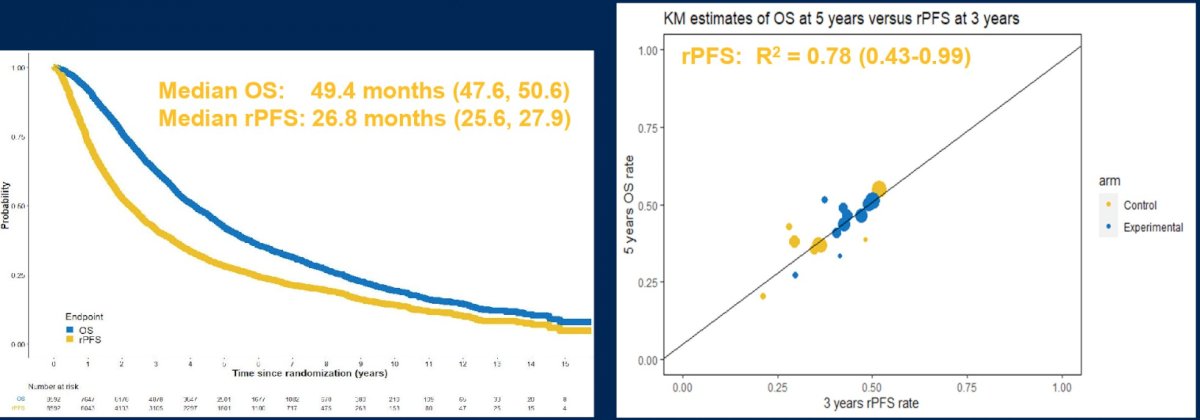
Dr. Szmulewitz’s conclusions for this abstract are as follows:
- rPFS and cPFS are strong intermediate surrogate endpoints for OS in mHSPC
- The strengths of the study are the large number of patients, patient-level data, and multiple randomized clinical trials
- The weaknesses of the study are that studies did not have uniform definitions of endpoints, and older studies do not have prognostic variables (ie. volume, de novo)
- Does this change clinical practice? Yes, the practice of clinical trial design. However, we need longer follow-up with the newer studies, and perhaps we can get access to industry data with uniform endpoints
So, are there any answers to our 5 mHSPC questions? First, do all patients with mHSPC benefit from ADT intensification? Dr. Szmulewitz emphasizes that yes, ADT alone is not sufficient. Based on ENZAMET, enzalutamide provides a meaningful benefit, and SHR3680 works as well, but there are still questions with regards to patients with visceral metastases, those with poor performance status (perhaps we will get answers from the PEACE 6-vulnerable study NCT04916613), underrepresented minorities, and world-wide cost.
Second, when should we use “triplet” (ADT + ARSI + docetaxel)? Dr. Szmulewitz notes that we still do not know. In ENZAMET, if we are using docetaxel for de novo metastatic disease then yes there is a benefit of triplet therapy (which is the same when using abiraterone in PEACE-1 or darolutamide in ARASENS). Currently the value of docetaxel addition to ARSI is not known.
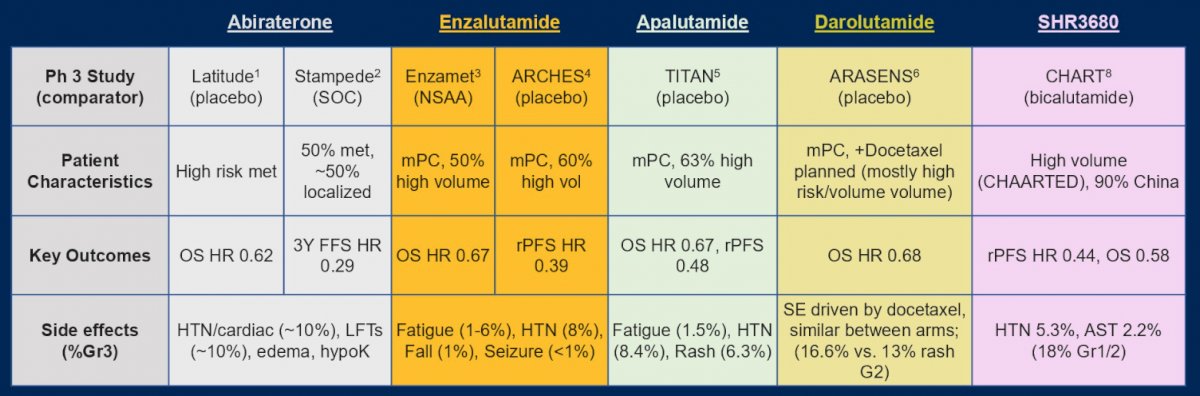
Third, how do we choose the right ARSI “intensifier”? Dr. Szmulewitz notes that this is up to the treating physician, which may include taking into account side effects, costs, and insurance coverage:
Fourth, is there any role for molecularly targeted precision medicine? Not yet, however there are several key trials ongoing:
- PSMA+ (PSMAddition NCT04720157)
- PTEN Loss (CAPItello NCT04493853)
- DDR mutated (AMPLITUDE NCT04497844, TALAPRO-3 NCT04821622)
Fifth, how can we accelerate innovation? Based on the intermediate clinical endpoints study, surrogate endpoints are key to marking early success. ARSI intensification + taxane (when needed) + molecularly targeted + informative intermediate endpoints leads to new and better options for mHSPC patients.
Presented by: Russell Z. Szmulewitz, MD, The University of Chicago, Chicago, IL
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 3 – Mon, June 7, 2022.


