(UroToday.com) Following three abstract presentations examining treatment approaches using theranostic treatment with 177Lu-PSMA-617 for metastatic castration-resistant prostate cancer (mCRPC) in the oral abstract session focused on Prostate, Testicular, and Penile cancers at the 2022 American Society of Clinical Oncology Annual Meeting held in Chicago and virtually, Dr. Susan F. Slovin provided a discussion of these data.
Dr. Slovin first made note of the assigned title “The Future is Now”, emphasizing that this alludes to work from the author William Gibson. In the original context, this implies that things that will constitute the norm in the future already exist for some today. Thus, we may interpret this to mean that there are implied inequalities: “the future has arrived – it just hasn’t been equally distributed yet”. She noted that this is particularly pertinent for the treatment of advanced prostate cancer given the disparate use of imaging and treatment approaches.
Given the recent introduction of 177Lu-PSMA-617 as a therapeutic option in advanced prostate cancer, Dr. Slovin emphasized the need to better understand which patients will benefit. In this context, there are no relevant serum biomarkers. Thus, there is significant interest in the interpretation of PSMA-PET imaging to guide patient selection. While this presentation focuses on 177Lu-PSMA-617 treatment, PSMA-PET imaging results may be important for immunotherapy and other treatment options including ADCs and BITEs. However, there is considerable uncertainty in terms of how to best operationalize these data. To this end, she highlighted recent work from Dr. Eiber and colleagues to define consistent and objective criteria for assessment PSMA-ligand PET/CT using the Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE) criteria.

Further, there are considerations regarding the prior treatment history in terms of suitability for 177Lu-PSMA-617 therapy. Finally, she emphasized the importance of remembering heterogeneity in the biologic framework of the tumor, noting that not all clones are equivalent in terms of PSMA expression. To this end, she highlighted an example of a patient under her care who underwent staging PSMA PET/CT with an intact prostate demonstrating a lack of PSMA avidity, despite biopsy proven prostate cancer.
Given this background, she moved to discussing data from TheraP as presented by Dr. Hofman at today’s ASCO meeting. TheraP is a randomized controlled comparison of 177Lu-PSMA-617 and cabazitaxel among men with mCRPC who had previously received docetaxel. While this study was designed with the primary outcomes of PSA response, these updated data focused on the key secondary endpoint of overall survival. Dr. Slovin emphasized the ability of investigators to follow their patients longitudinally while capturing the use of post-protocol therapies. In the intention to treat the population, she noted that prior publications of the TheraP cohort (based on the data from 20 months) were highly suggestive that overall survival would be improved. However, these updated data show no difference in overall survival between patients treated with 177Lu-PSMA-617 or cabazitaxel.

However, she further considered the question of whether we can better identify patients for treatment with 177Lu-PSMA-617, considering PSMA as a biomarker. Based on data presented by Dr. Hofman from the TheraP cohort, PSMA SUVmean of 10 or greater is strongly associated with a greater benefit from 177Lu-PSMA-617, compared to cabazitaxel. Thus, given the proven benefits here of 177Lu-PSMA-617, Dr. Slovin noted that clinicians must choose among a sea of life prolonging therapies for patients with mCRPC. Certainly, 177Lu-PSMA-617 has demonstrated equivalent long-term survival to cabazitaxel with improved response rates and better tolerability. Thus, she concluded that 177Lu-PSMA-617 is a reasonable treatment. However, we need to think further about when best to utilize this approach, considering questions of disease burden and prior lines of therapy. She further highlighted pragmatic questions of access, cost, and real-world uptake of both PSMA-based imaging and radioligand therapy.
Building on this, she transitioned to a discussion of the presentation from Dr. Vaishampayan which examined a post hoc exploratory analysis of the VISION trial considering prior treatments (before randomization) and concurrent treatments (as part of the standard of care) for patients randomized to 177Lu-PSMA-617 or not. In terms of takeaways, she highlighted that 177Lu-PSMA-617 provided a consistent benefit that did not appear to be mediated in a meaningful way by prior therapies received or concurrent treatment given as part of standard of care. However, long-term consequences are unknown.
Finally, she noted that Dr. Armstrong brings these concepts together in his presentation of another VISION sub-study examining the ability of quantitative imaging analyses of 68Ga-PSMA-11 PSMA/PET imaging for patients treated with 177Lu-PSMA-617 and standard of care in the VISION trial.

She noted that these analyses demonstrated that whole body SUVmean PSMA uptake, when operationalized either in quartiles or continuously, was strongly associated with both radiographic progression-free survival and overall survival as noted in the figures below.
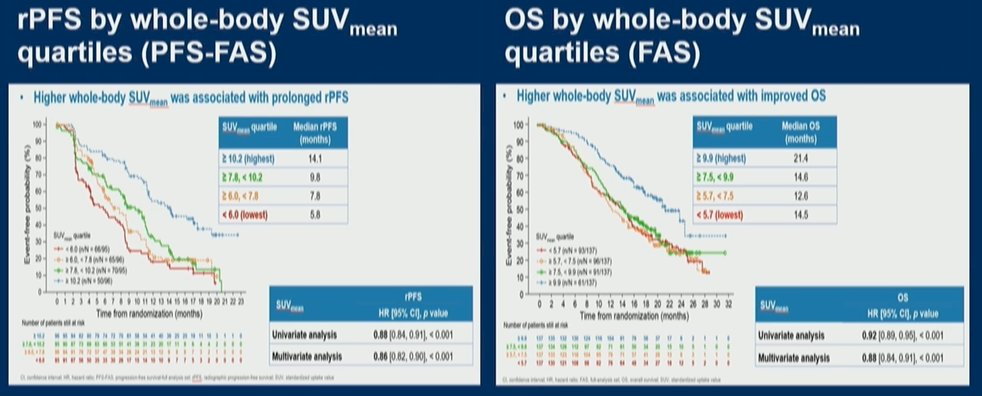
Thus, Dr. Slovin concluded that for men treated with 177Lu-PSMA-617, a higher whole body SUVmean was strongly and clinically meaningfully associated with improved long-term clinical outcomes. Further, patients without PSMA positive disease in the liver or bone had improved outcomes as well.
Taking all of these presentations together, she noted that all three underscore the need to refine and stratify eligibility for use of PSMA-based radioligand therapy. The PSMA-based radiographic criteria proposed by Dr. Kuo and presented by Dr. Armstrong provide a framework for identifying and stratifying patients to benefit from therapy. This approach may inform future nomograms. However, she noted numerous pragmatic and logistic issues to applying these data in the real world, including differences in practice between community and academic centers, and the availability of both PSMA-based imaging and nuclear medicine support for theranostic therapy. Thus, there is likely an increasing need for travel to centers of excellence in addition to considerations regarding regulatory and reimbursement issues.
Thus, given these issues, she noted that the future may still be “the future”, rather than “now”. To make this leap, there will need to be significant advancements in terms of funding, validation of targets, and supply chain considerations. However, these data clearly support the need for interdisciplinary collaborations to care for patients with advanced prostate cancer.
Presented by: Susan F. Slovin, MD, PhD, Medical Oncologist, Associate Vice Chair, Academic Administration, Department of Medicine, Memorial Sloan Kettering Cancer Center


