(UroToday.com) At the 2022 American Society of Clinical Oncology Annual Meeting held in Chicago and virtually, the oral abstract session focused on Kidney and Bladder cancers on Friday afternoon included a presentation from Dr. Thomas Powles describing results of the CALYPSO trial, a three-armed comparison of durvalumab either alone or in combination with either savolitinib or tremelimumab in patients with advanced renal cell carcinoma (RCC) who had progressed on prior systemic therapy.
There have been significant advances in the treatment of advanced RCC in the past five years, including the introduction of immune checkpoint inhibitor monotherapy in the second-line setting followed by immune checkpoint-based doublet therapies in first-line treatment. However, despite these new treatment approaches, few patients are cured, and novel treatment approaches are still needed. Potential treatment options include MET inhibition with savolitinib or CTLA-4 inhibition with tremelimumab. In CALYPSO (NCT02819596), the authors examined these treatment approaches as monotherapy or in combination with the PD-L1 inhibitor durvalumab.
To do so, they performed a multinational open-label randomised phase II study. They included patients with RCC, who had previously received VEGF targeted therapy but had not received immune checkpoint inhibitors or MET inhibitors.
Patients were randomized to one of four arms initially: durvalumab, savolitinib, durvalumab + tremelimumab or durvalumab + savolitinib. Due to a lack of efficacy, the savolitinib arm was closed early.
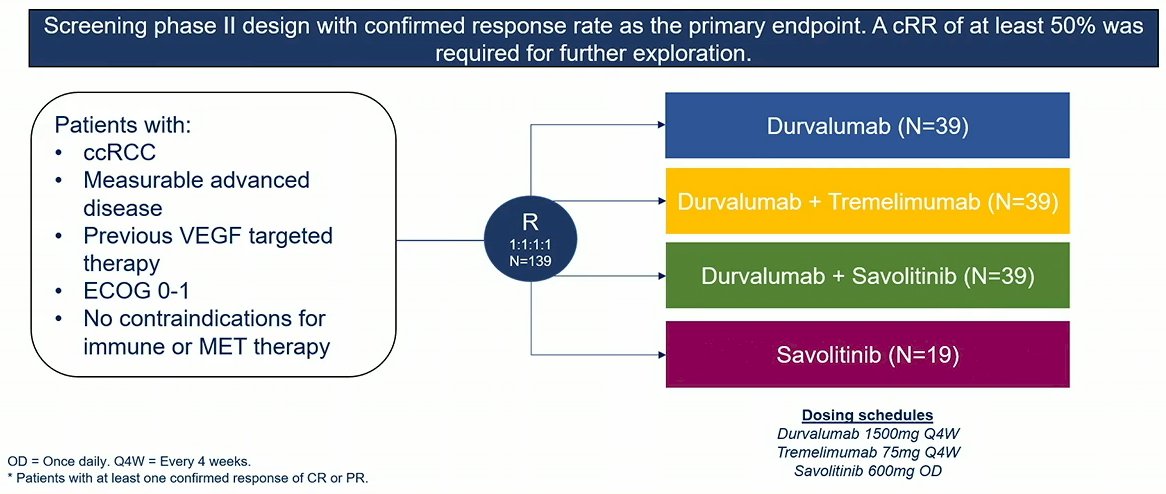
In this phase II trial, the primary endpoint was confirmed response rate (cRR). A response rate of at least 50% was required for further exploration. DNA alterations were measured using Foundation One and PD-L1 analysis was performed with SP263. In his presentation at this year’s ASCO meeting, Dr. Powles provided data from the pre-planned 12-month interim analyses after the cohort completed randomisation.
Between 2017 and 2021, the authors randomized a total of 139 patients of whom 39 received durvalumab, 22 received savolitinib prior to the arm closing, 39 received the combination of durvalumab + tremelimumab, and 39 received durvalumab + savolitinib. The median age of included patients, in keeping with this study population, was 62 years (range: 28 – 85).
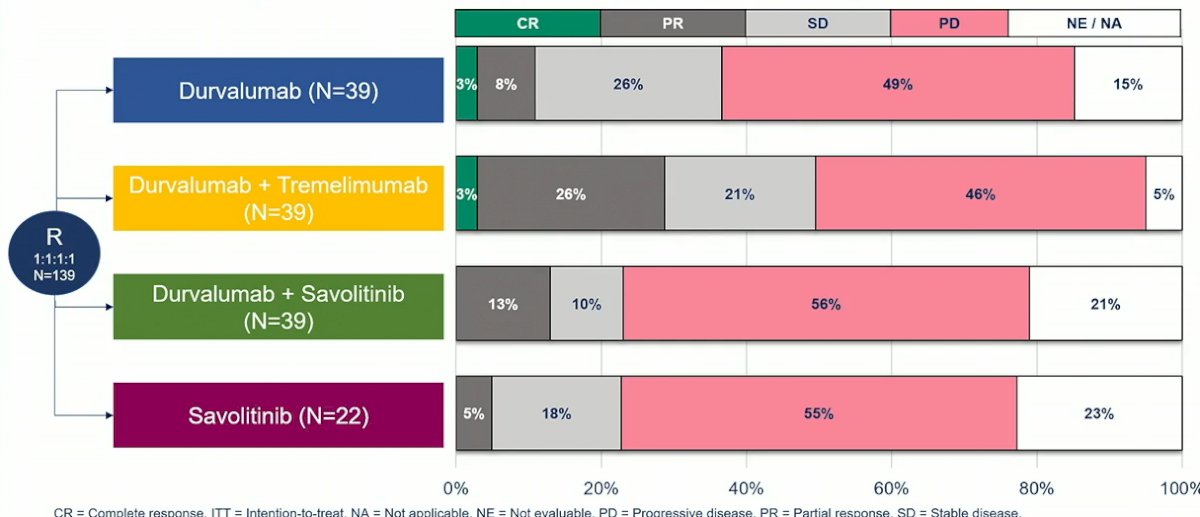
The confirmed response rate (cRRs) was 10% among patients receiving durvalumab monotherapy, 5% among those receiving savolitinib monotherapy, 28% among those receiving the combination of durvalumab + tremelimumab, and 13% among those receiving the combination of durvalumab + savolitinib. This failed to meet the primary study objective.

Considering all treatment outcomes, Dr. Powles noted that progressive disease was the most commonly observed outcome for patients in this trial across all four arms.
In addition to the clinical outcome assessment, the authors assessed DNA alterations using next generation sequencing, including VHL, PBRM1, SETD2, and BAP1.
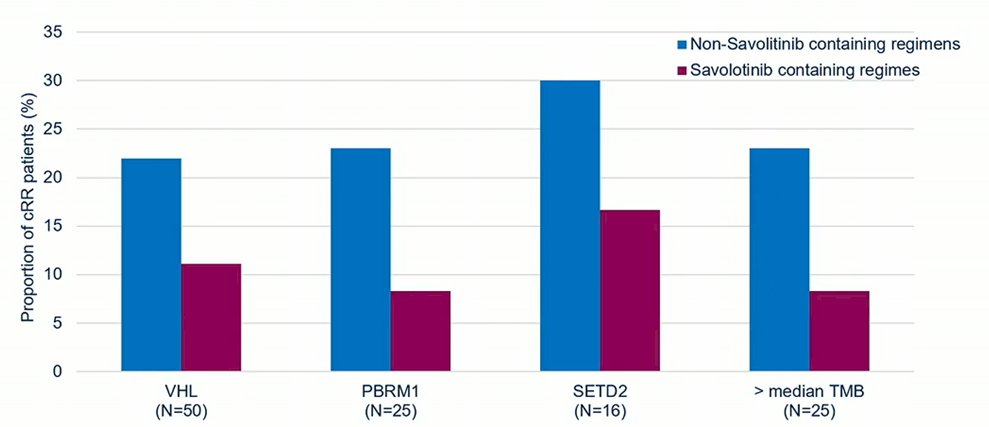
Among a subset of 17 patients with MET-driven tumors, cRRs were similarly low to the overall cohort: 0% among those receiving durvalumab (0/7) or savolitinib (0/2) monotherapy, 50% among those receiving the combination of durvalumab + tremelimumab (1/2), and 17% among those receiving the combination of durvalumab + savolitinib (1/6). Thus, despite the MET-pathway targeting mechanism of action of savolitinib, there did not appear to be a dramatic clinical benefit for savolitinib including regimes for these patients.

In non-MET driven tumors, there appeared to be comparable outcomes for patients treated with savolitinib containing and non- savolitinib containing regimes. When stratified by specific DNA alterations, savolitinib containing regimes appeared to be somewhat inferior to the alternative regimes in this trial.
In patients with PD-L1 positive tumors, cRRs were 14% (1/7) with the combination of durvalumab + tremelimumab and 33% (2/6) for durvalumab monotherapy. Among those patients who received regimes containing immune checkpoint inhibitors, PD-L1 positive patients appeared to fare somewhat better than those who were PD-L1 negative though, given this small sample size, this was not significant.
In terms of secondary outcomes, 12-month progression-free survival (PFS) rates were 26% (80% confidence interval [CI]: 17% - 36%) for patients treated with durvalumab, 21% (80% CI: 10% - 35%) for those who received savolitinib, 33% (80% CI: 24% - 43%) for those randomized to durvalumab + tremelimumab, and 17% (80% CI: 10% - 26%) for those randomized to durvalumab + savolitinib. The median overall survival was relatively comparable between treatment arms: 26.1 (80% CI: 16.2 – 32.0) months in those in the durvalumab arm, 23.1 (80% CI: 20.6 – 29.7) months for those in the savolitinib arm, 21.9 (80% CI: 16.3 – 31.5) months for those in the durvalumab + tremelimumab arm, and 16.1 (80% CI: 10.3 – 18.8) months for those in the durvalumab + savolitinib arm.
In terms of safety, there was one patient receiving the durvalumab + tremelimumab who died as a result of treatment. Of the 136 patients who received treatment, grade 3 or more treatment related adverse events occurred in 10% (4/39) of those receiving durvalumab, 26% (5/19) of those receiving savolitinib, 23% (9/39) of those receiving durvalumab + tremelimumab, and 23% (9/39) of those receiving durvalumab + savolitinib.

Thus, Dr. Powles concluded that these randomized phase II data do not show significant efficacy of either savolitinib monotherapy or in combination with durvalumab in RCC, despite being safe and tolerable. Furter, the addition of tremelimumab to durvalumab did not demonstrate clearly superior efficacy to durvalumab monotherapy.
Despite CALYPSO being negative among these patients with clear cell histology, Dr. Powles emphasized the potential of the combination of savolitinib + durvalumab in patients with papillary renal cell carcinoma.
Presented by: Thomas Powles, MD, PhD, FCRP, Barts ECMC, Barts Cancer Institute, Queen Mary University of London, London, United Kingdom

