(UroToday.com) At the 2022 American Society of Clinical Oncology Annual Meeting held in Chicago and virtually, the oral abstract session focused on Kidney and Bladder cancers on Friday afternoon included a presentation from Dr. Cristina Suárez examining the association between the depth of response and clinical outcomes in patients treated with nivolumab and cabozantinib as first-line therapy for advanced renal cell carcinoma (RCC) in the context of the CheckMate 9ER trial.
The CheckMate 9ER trial (NCT03141177) demonstrated that patients receiving first-line treatment with the combination of nivolumab and cabozantinib had significantly improved progression-free (hazard ratio 0.56) and overall survival (0.70) compared to those who received sunitinib for advanced and metastatic renal cell carcinoma (RCC). Further, patients receiving the combination of nivolumab and cabozantinib were twice as likely to have an objective or complete response.
In this abstract, the authors examined the association between the depth of response and clinical outcomes with extended follow-up (minimum 25.4 months; median 32.9 months).
While previously described and published, to briefly summarize, the CheckMate-9ER trial randomized patients to receive either nivolumab (240 mg) every 2 weeks plus cabozantinib (40 mg) once daily or sunitinib (50 mg once daily; 4 weeks of each 6-week cycle).

In this analysis, the authors categorized patients into depth of response subgroups based on the best overall response according to blinded independent central review [BICR] per RECIST v1.1 and best tumor reduction threshold. Thus, there were six groups: complete response (CR); partial response subdivided by a tumor reduction of ≥80%–<100% (PR1); ≥60%–<80% (PR2); or ≥30%–<60% (PR3); stable disease (SD); and progressive disease (PD).

Following a 6-month post-randomization landmark, the authors examined PFS (per BICR) and OS, stratified by depth of response subgroups. The authors further assessed for differences in treatment-related adverse events (TRAEs) between these subgroups.
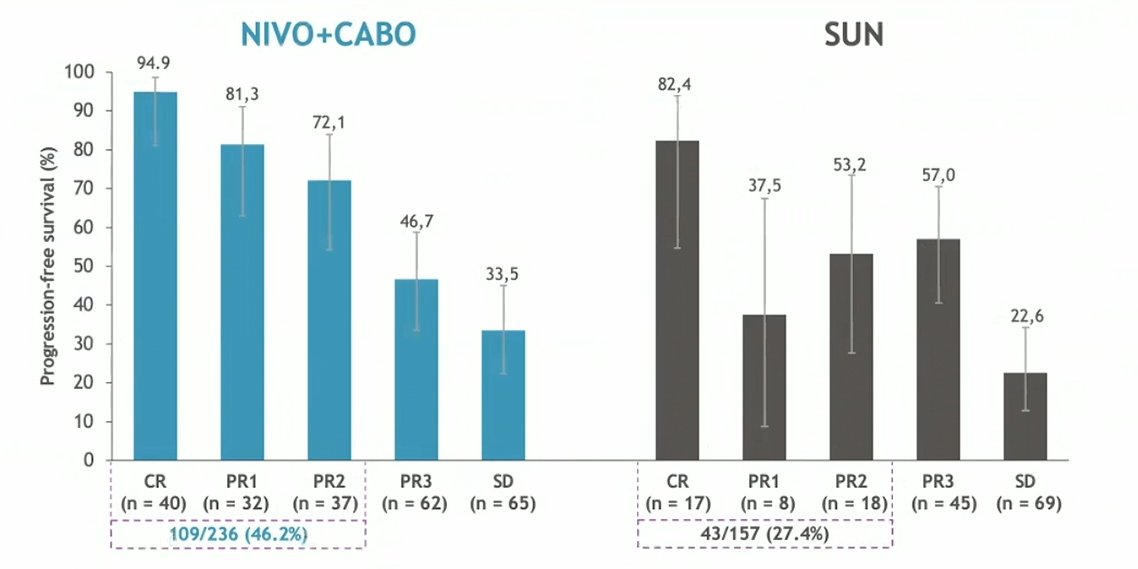
Among the 323 and 328 patients randomized to nivolumab and cabozantinib or sunitinib, respectively, 236 and 157 were progression-free and alive, respectively, and 293 and 253 were alive, respectively, at 6-months following randomization to allow for assessment and categorization of their depth of response. Overall, a larger proportion of patients randomized to nivolumab and cabozantinib had deeper responses (ie. CR, PR1 or PR2) to treatment, compared those randomized to sunitinib.

Deeper responses to nivolumab and cabozantinib were associated with improved 12-month progression-free survival compared to responses observed with sunitinib: among those with CR at 6-month (94.9% vs 82.4%), those with PR1 at 6-months (81.3% vs 37.5%), and PR2 at 6-months (72.1% vs 53.2%).
While increasingly deeper responses were associated with improved overall survival regardless of treatment arm, there were no differences in overall survival between those patients randomized to nivolumab and cabozantinib or sunitinib when stratified by their initial depth of response (CR, PR1, PR2, and PR3).

Interestingly, patients who achieved CR or PR1 responses had equivalent outcomes regardless of treatment arm.
Finally, the authors did not identify any meaningful association between depth of response and treatment-related adverse events in either treatment arm.

Thus, they concluded that, in this exploratory analysis of the CheckMate 9ER trial, a greater proportion of patients randomized to nivolumab and cabozantinib achieved a deep response compared to those receiving sunitinib. A deeper response was generally associated with improved progression-free and overall survival independent of treatment received. These results, however, suggest that depth of response may be a useful indicator for durable efficacy and improved prognosis.
Presented by: Cristina Suárez, MD, Vall d’Hebron University Hospital, Barcelona, Spain

