(UroToday.com) The 2022 ASCO annual meeting featured an oral abstract session on kidney and bladder cancer, including a discussant presentation by Dr. Michiel S. Van Der Heijden discussing expanding horizons in localized bladder cancer. Dr. Van Der Heijden discussed “Cell-free DNA methylation as a predictive biomarker of response to neoadjuvant chemotherapy for patients with muscle-invasive bladder cancer in SWOG S1314” by Dr. Thomas Flaig, “TRUCE-02: An open label, single-arm, phase II study of tislelizumab combined with nab-paclitaxel for high-risk non-muscle-invasive urothelial bladder carcinoma” by Dr. Zesheng An, and “Final clinical results of pivotal trial of IL-15RαFc superagonist N-803 with BCG in BCG-unresponsive CIS and papillary NMIBC” by Dr. Karim Chamie.
Dr. Van Der Heijden started by emphasizing that the current “standard” to beat in BCG-refractory CIS is pembrolizumab. This is based on the KEYNOTE-057 trial that demonstrated a 1 year complete response rate of 19% among men with BCG-refractory disease.1 In the QUILT 3.032 trial, all treated patients received intravesical N-803 plus BCG, consistent with the standard induction/maintenance treatment schedule. The primary endpoint for Cohort A (CIS) is incidence of complete response of CIS at any time. The primary endpoint for Cohort B (Papillary) is disease-free rate at 12 months. The trial design for QUILT 3.032 is as follows:
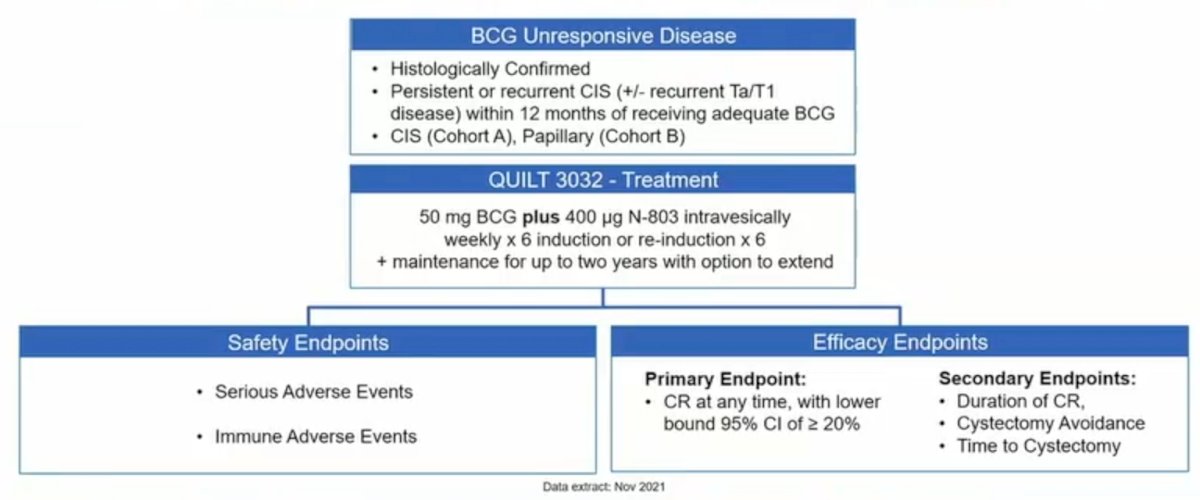
Among CIS patients, the complete response rate was 71%, with a complete response rate at 12 months of 45%. Dr. van der Heijden notes that this in comparison to KEYNOTE-057 with complete response rates of 41% and 19%, respectively. Furthermore, among patients with papillary disease in QUILT 3.032, the median DFS was 23.6 months, the DFS rate at 12 months was 57%, and the DFS rate at 24 months was 48%. This meets the given standard for these trials of 30% DFS rate at 12 months.
Discussing the TRUCE-02 trial, Dr. Van Der Heijden notes that this trial included patients with high-risk NMIBC (T1, HG-Ta, CIS) or unresectable tumor at TURBT. Interestingly, there was no BCG requirement for this trial. The trial schema for TRUCE-02 is as follows:
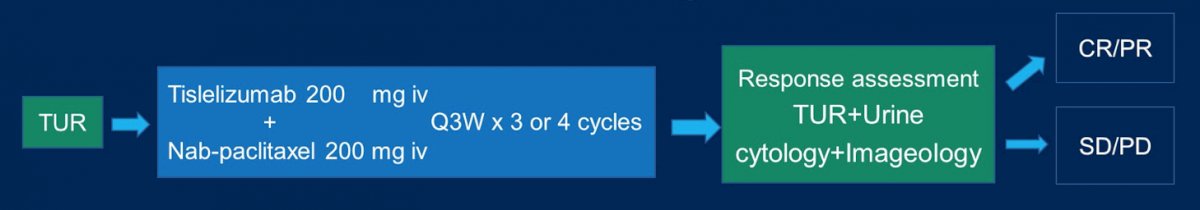
Among 55 patients enrolled in the trial, there was a median follow-up of 9 (range 3-16) months. Overall, 42 patients completed 3 or 4 treatment cycles and reached the primary endpoint. There were 23 patients that achieved a complete response (55%, 95% CI 43.6% to 74.4%), and the ORR was 60% (95% CI 45.2% to 74.8%). As a secondary endpoint, 28 patients remained cystectomy-free (66.7%) and the 12-month cystectomy-free survival rate was 62.7%. As follows is the Kaplan Meier curve for cystectomy-free survival:

Dr. Van Der Heijden’s conclusions for the two NMIBC trials are as follows:
- QUILT-3.032:
- These are encouraging results for Cohort A with BCG-refractory CIS
- Cohort B (papillary) results are exploratory
- Randomized phase 3 results are needed for clinical implementation
- TRUCE-02:
- The rationale and study design for this trial are problematic: there was no BCG requirement, there is a mix of CIS and papillary disease, and there is no standardized assessment schedule
- The results appear insufficient to justify systemic chemotherapy
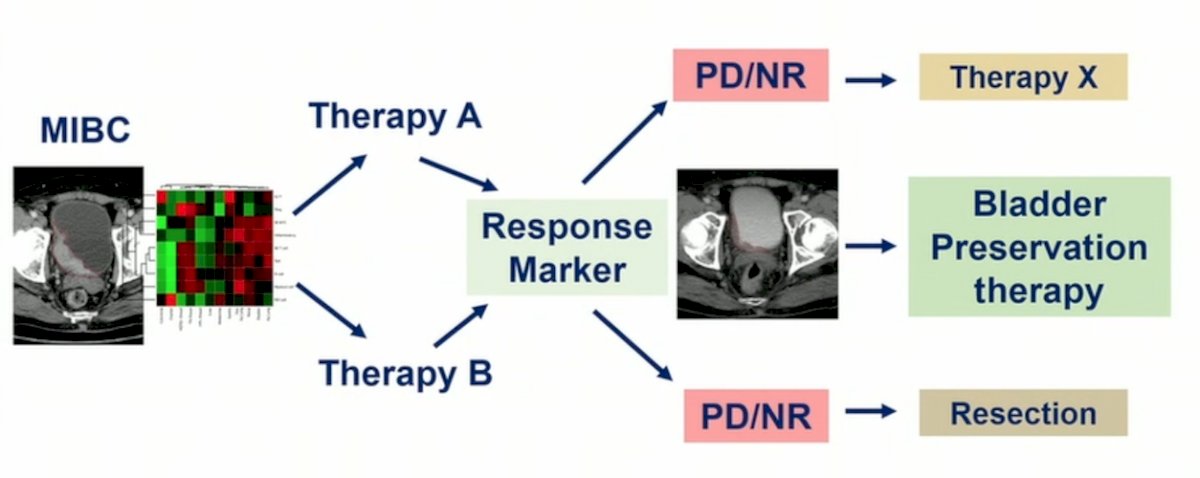
Dr. Van Der Heijden then discussed the cfDNA methylation assessment of response to neoadjuvant therapy study using data from the SWOG S1314 trial. According to Dr. Van Der Heijden, a model for ctDNA predicting response to neoadjuvant chemotherapy for patients with MIBC may look like the following:
The SWOG S1314 trial schema is as follows:
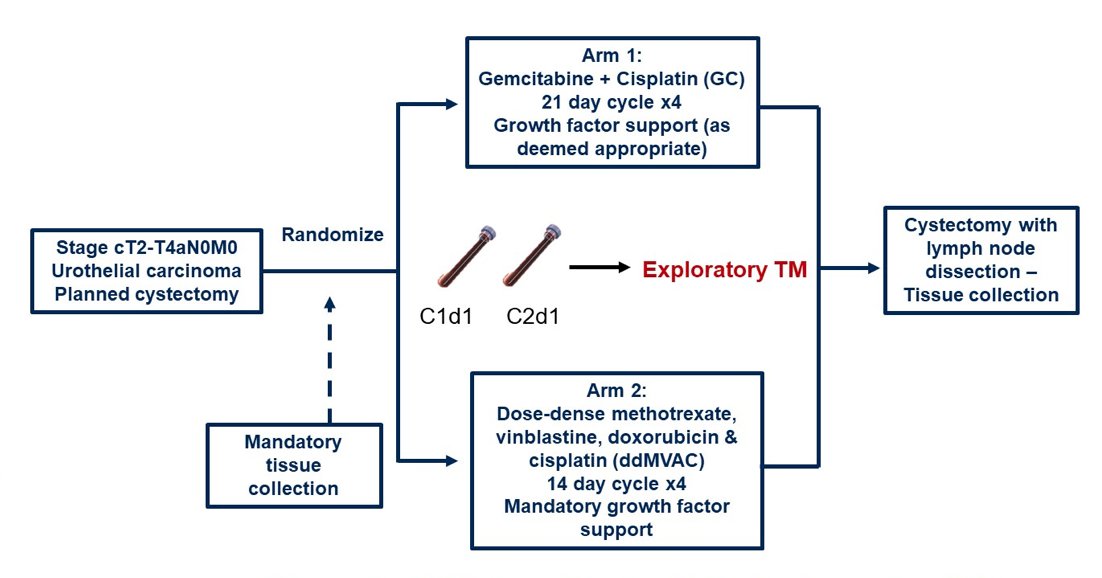
In this study, blood samples were collected prospectively from 72 patients before and during standard neoadjuvant chemotherapy. At radical cystectomy, pathologic response was documented, and plasma cell-free DNA was profiled using Infinium MethylationEPIC BeadChip array. This study found that there was no single methylation locus statistically correlated with response after adjustment for multiple comparisons (>850K probes in Infinium chip). However, on tSNE mapping, responders and non-responders clustered separately based on the 500 most differentially methylated DNA loci. Thus, the Random Forest machine learning and leave-one-out cross validation to all 850K loci was applied to create the methylation-based response score (mR-score). By using pre-chemotherapy plasma cell-free DNA, they developed a mR-score predictive of pathologic response:
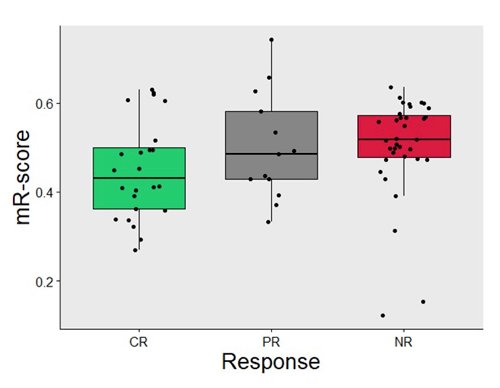
Furthermore, the mR-score was independent of bladder cfDNA quality. However, Dr. Van Der Heijden wonders what the source of the signature is?
Dr. Van Der Heijden concluded his presentation discussing expanding horizons in localized bladder cancer with the following take-home messages:
- Localized bladder cancer still has a poor prognosis
- There are many new drugs coming, including immunotherapy ADCs, and FGFR inhibitors
- Biomarkers are needed for therapy selection
- Early response prediction is necessary to allow for switching treatment
- cfDNA methylation is interesting, but requires further research
Presented by: Michiel S. Van Der Heijden, MD, PhD, Netherlands Cancer Institute, Amsterdam, The Netherlands
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 3 – Mon, June 7, 2022.
References:


