(UroToday.com) The 2022 ASCO annual meeting featured an oral abstract session on kidney and bladder cancer, including a presentation by Dr. Robert Jones discussing the final analysis of the ATLANTIS trial, a randomized, double blind, phase II clinical trial of maintenance cabozantinib following chemotherapy for metastatic urothelial carcinoma. Until January 2021, standard treatment for advanced urothelial carcinoma was a combination platinum-based chemotherapy followed by surveillance until progression. Indeed, platinum-based chemotherapy is an active first line therapy in metastatic urothelial carcinoma, but duration of response is usually short. Cabozantinib is a tyrosine kinase inhibitor for VEGFR, AXL, MET and RET which has shown some clinical activity in platinum-pretreated progressive urothelial carcinoma. Dr. Jones and colleagues hypothesized that switch maintenance therapy with the tyrosine kinase inhibitor cabozantinib, in patients who have derived clinical benefit from platinum-based chemotherapy, would improve outcomes for metastatic urothelial carcinoma.
ATLANTIS is an adaptive, multi-comparison, phase II trial platform. It tests multiple maintenance therapies for metastatic urothelial carcinoma patients who complete 4 to 8 cycles of platinum-based chemotherapy without disease progression:
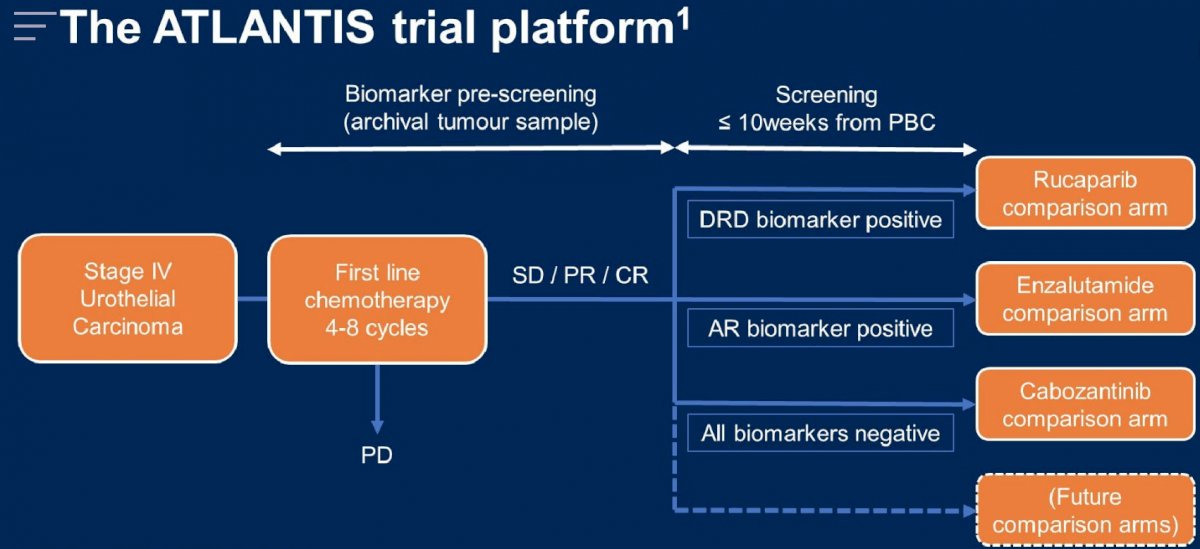
While some patients were selected for randomization into alternative comparisons in the platform based on putative predictive biomarkers, those who were not selected were randomised 1:1 to commence either cabozantinib 40mg once-daily or matching placebo within 10 weeks of completing platinum-based chemotherapy until progression. The cabozantinib comparison arm trial design is as follows:
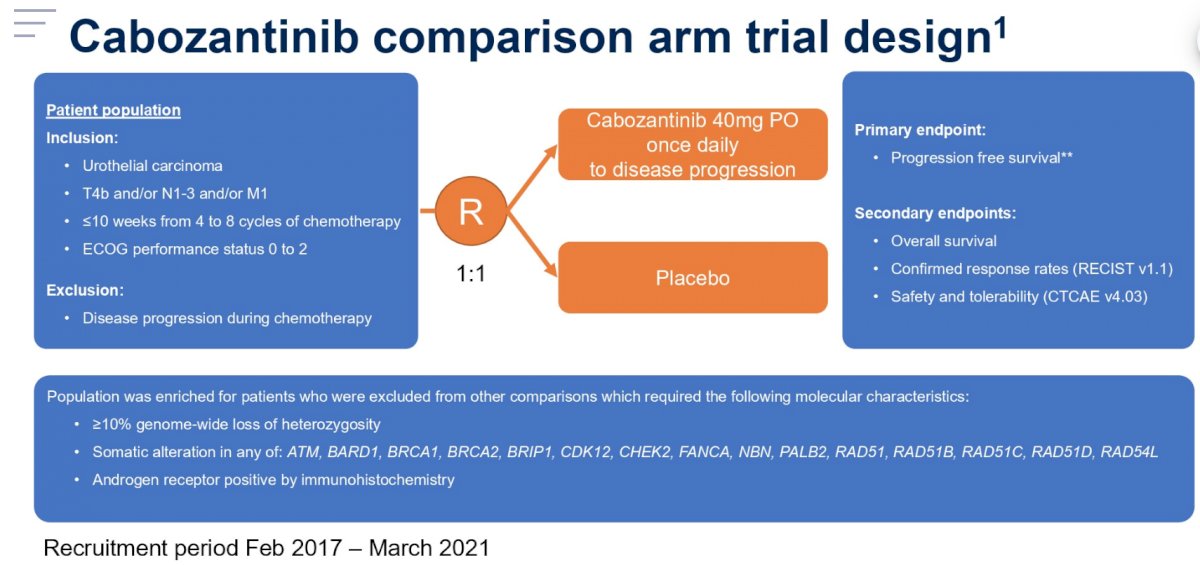
The primary endpoint was progression free survival (PFS) and 114 events in 140 patients (90% power, 20% 1-sided level alpha) were required to target a hazard ratio of 0.67. Due to a change in standard of care (maintenance avelumab),1 this was modified to 50 events in 57 patients (72.3% power, 20% 1-sided alpha) prior to the study closing early. PFS was compared between trial arms, by intention to treat, within a Cox model incorporating baseline minimization factors. Secondary endpoints included overall survival (OS), response rate, maximum percentage decrease in measurable disease, safety and tolerability.
There were 30 patients were randomised to cabozantinib and 31 to placebo from 25 sites (February 2017-March 2021). Patient characteristics, including a median age of 69.0 years, 75.4% male, 75.4% bladder primary, 67.2% ECOG PS 0, 70.5% prior cisplatin, and 36.1% with visceral metastases, were balanced by treatment arm. There were 25 (83.3%) PFS events in the cabozantinib arm and 26 (83.9%) in the placebo arm. The median PFS was 13.7 weeks (80% CI 12.1-23.3) with cabozantinib and 15.8 weeks (80% CI 11.3-23.6) with placebo (adjusted HR 0.89 favoring cabozantinib, 80% CI 0.61-1.3, 1-sided p = 0.35):

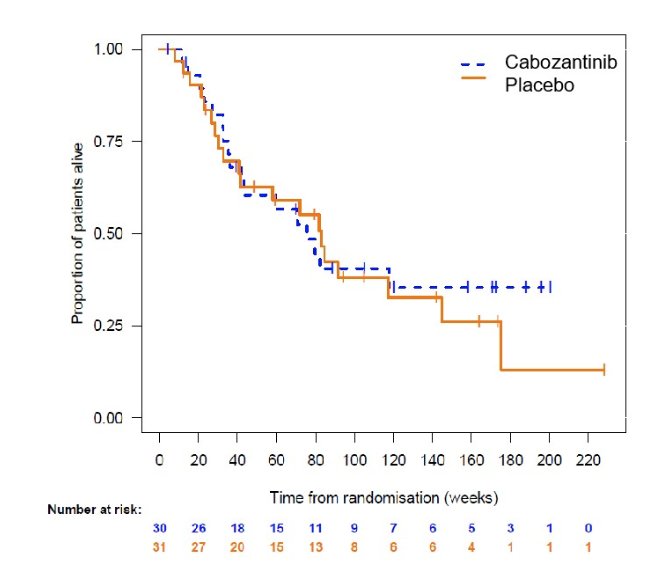
There was also no difference in overall survival (HR 0.80 (0.52-1.23; p=0.25):
In the safety population (n = 61) treatment related adverse events were mostly low grade. The most frequent (all grades) events, fatigue (56.7% vs 32.2%, p = 0.02), hypertension (43.3% vs 12.9%, p = 0.01), nausea (30% vs 19.4%, p = 0.36) and diarrhea (40.0% vs 6.5%, p < 0.01), were more common with cabozantinib. Overall, cabozantinib was tolerable with median duration of treatment of 13 28-day cycles of cabozantinib vs 10 of placebo. There were 13 (43.%) patients vs 3 (9.7%) patients that required dose reduction in the cabozantinib and placebo arms respectively.
Dr. Jones concluded his presentation of the final analysis of the ATLANTIS trial, a randomized, double blind, phase II clinical trial of maintenance cabozantinib following chemotherapy for metastatic urothelial carcinoma with the following take-home messages:
- Though underpowered, this study does not support further investigation of cabozantinib alone as a maintenance therapy after platinum-based chemotherapy in unselected patients with advanced urothelial cancer
- Negative patient selection for DRD and androgen receptor biomarkers may bias interpretation
- Placebo is no longer an acceptable control arm in this indication
- Future trials should consider combining novel agents with maintenance immunotherapy
Presented By: Robert J. Jones, MD, University of Glasgow, Glasgow, United Kingdom
Co-Authors: Syed A. Hussain, Alison Jane Birtle, Yee Pei Song, Deborah Enting, Guy Faust, Serena Hilman, Satinder Jagdev, Ursula Brigid McGovern, Omi Parikh, Amy Lewis, Eileen Soulis, Avril Trevethan, Simon J. Crabb, Thomas Powles
Affiliations: University of Sheffield and Sheffield Teaching Hospitals, Sheffield, United Kingdom, Rosemere Cancer Centre, Royal Preston Hospital, Preston, United Kingdom, The Christie Hospital NHS Foundation Trust, Manchester, United Kingdom, Guy's and St. Thomas' NHS Foundation Trust, London, United Kingdom, Oncology Department, Leicester, United Kingdom, University Hospital Bristol NHS Foundation Trust, Bristol, United Kingdom, Leeds Cancer Centre, Leeds, United Kingdom, University College Hospital, University College London Hospitals NHS Foundation Trust, London, United Kingdom, Royal Preston Hospital, Preston, United Kingdom, Queens Hospital, Romford, United Kingdom, CRUK Glasgow Clinical Trials Unit, University of Glasgow, Glasgow, United Kingdom, University of Southampton Faculty of Medicine, Durley, United Kingdom, Barts Cancer Institute, Cancer Research UK Experimental Cancer Medicine Centre, Queen Mary University of London, Royal Free National Health Service Trust, London, United Kingdom
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 3 – Mon, June 7, 2022.
References:


