(UroToday.com) In this presentation, Dr. Garcia del Muro presented data from the IMMUNOPRESERVE-SOGUG trial, which aimed to test combined immunotherapy with durvalumab and tremelimumab and radiotherapy as a chemotherapy-free strategy for bladder preservation in muscle invasive bladder cancer (MIBC). The rationale for this approach is based on (1) immunotherapy has significant activity in the neoadjuvant and advanced setting for urothelial cancers and (2) the combination of radiation and dual checkpoint blockade can activate non-redundant immune mechanisms to generate anti-tumor immune activity.
This was a phase 2 trial, with the schema shown below. A safety-run in cohort of five patients was utilized to assess for dose limiting toxicities. Biomarker analysis is planned on peripheral blood from various time points to assess lymphocyte populations and cytokines, as well as on tumor biopsy for T-cell receptor clonality, PD-L1 expression and stromal analysis.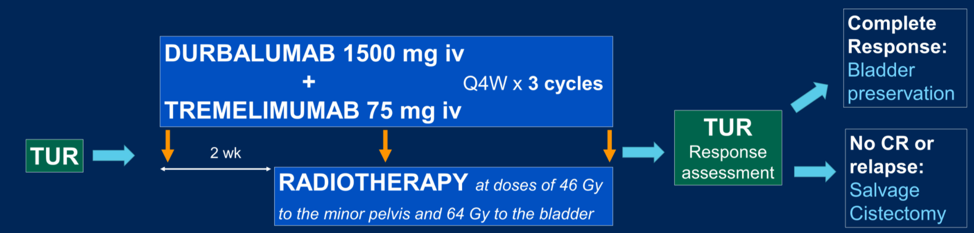
Patients eligible for the study were required to have urothelial MIBC, clinically node negative with tumor stage T2-T4a. Patients must either have refused radical cystectomy or be medically ineligible for the procedure and have no contra-indications to immunotherapy. The primary endpoint of the study was complete response in the intention to treat population, defined in this case as the absence of detectable MIBC at the time of post-treatment cystoscopy and tumor biopsy. A two-stage sequential design was utilized, assuming a null hypothesis of less than 50% complete responses and an alternate hypothesis of more than 70%. At a power of 80% and alpha of 0.1, 12 patients were required for the first stage, and if more than 6 CRs were observed, 20 more patients were to be enrolled.
A total of 32 patients were enrolled from 47 screened patients and were included in the safety and efficacy analysis. Patient characteristics are shown below.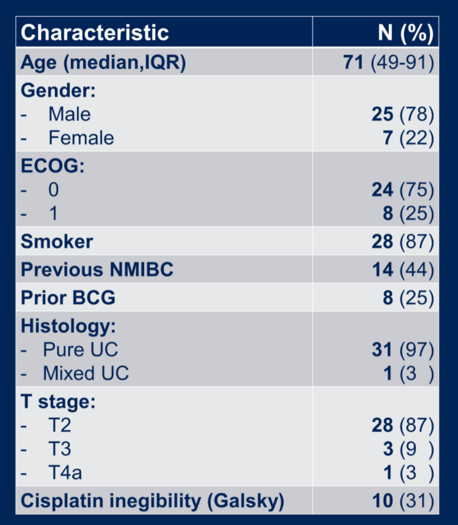
With regards to the primary endpoint of complete response, defined as less than or equal to pT1 disease at biopsy, 26 out of 32 patients (81%) met this endpoint, with 25 out of 26 patients having a pathologic complete response, and 1 patient having residual non-MIBC. Two patients had residual MIBC at the time of tumor biopsy, and 4 patients were not evaluable due to rejection, clinical impairment, covid19-related death and death from peritonitis. The median disease-free survival rate at 12 months was 76%, and the median overall survival at 12 months was 87%. The two patients who had residual MIBC at subsequent tumor biopsy underwent salvage cystectomy. The 12-month bladder-intact disease-free survival was therefore measured at 73%.
Treatment-related adverse events are shown below.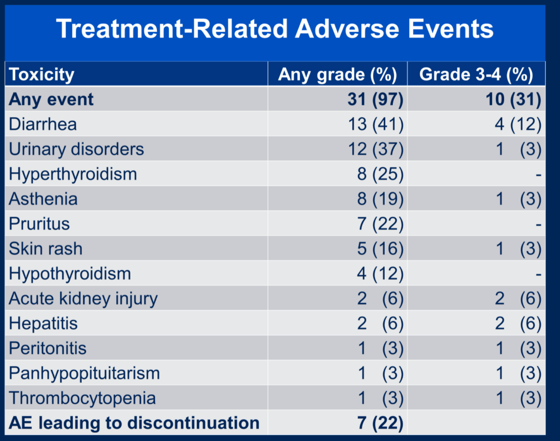
Though biomarker work is ongoing, preliminary data suggest upregulation of T-cell PD-1 expression, a decrease in the percentage of naïve T-cells, and the presence of more effector T-cells after immunotherapy and radiotherapy treatment.
Dr. Garcia del Muro concluded his talk by stating that combined modality bladder-preserving with combined immunotherapy (durvalumab and tremelimumab) and concurrent radiotherapy if feasible, safe, and has a high-rate of clinical response and bladder preservation at least at 12-month follow-up. Longer follow-up is required to confirm these benefits, and biomarker data may help understand the mechanisms underlying successful bladder preservation.
Presented by: Xavier Garcia del Muro, MD, PhD, medical oncologist at the institute catala d’oncologia hospitalet barcelona
Written by: Alok Tewari, MD, PhD, Medical Oncologist at the Dana-Farber Cancer Institute, at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Virtual Annual Meeting #ASCO21, June, 4-8, 2021


