(UroToday.com) Genomic characterization of metastatic castrate-resistant prostate cancer has identified commonly occurring alterations but also recurrently altered genes at much lower frequencies. In this presented poster, the authors aimed to evaluate the anti-tumor activity of novel targeted therapies in patients with metastatic castrate-resistant prostate cancer. For the purpose of this goal, the authors initiated an umbrella trial using circulating tumor DNA (ctDNA) to enrich accrual for cancers with alterations that may predict the response (NCT02905318, NCT03385655).
The study design is shown in Figure 1, and this is an ongoing, open-label, multicenter, multi-arm, two-stage phase two trial of metastatic castrate-resistant prostate cancer patients being enrolled and stratified to treatment arms according to their biomarker status.
Figure 1 – Study design: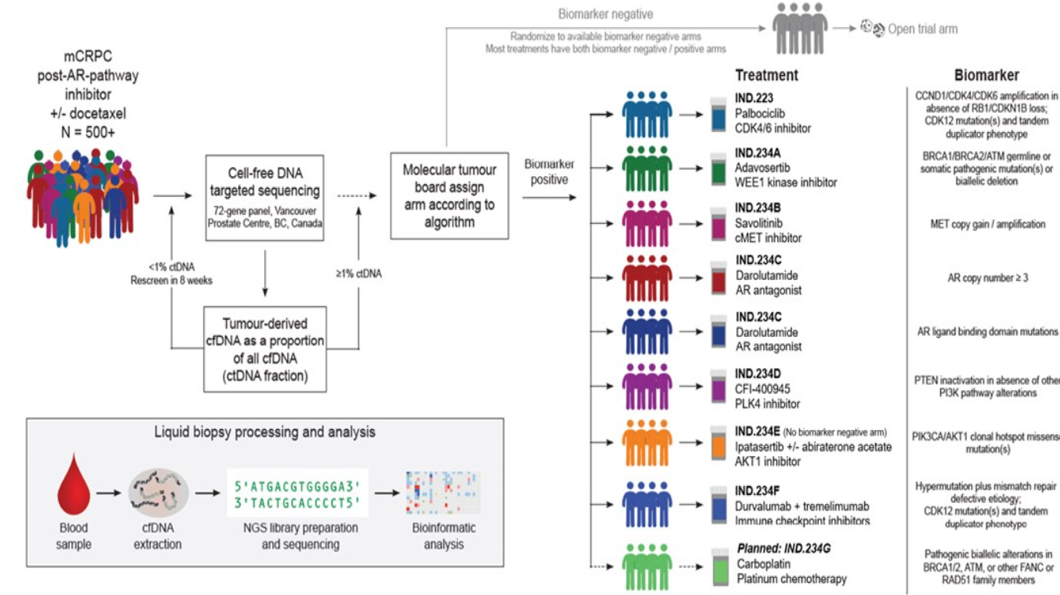
All patients had a baseline plasma-derived cell-free DNA subjected to targeted sequencing, and patients were allocated to a specific treatment arm by a tumor board, based on priority criteria or by randomization if the biomarker was negative. Patients had to be 18 years or older with an ECOG performance status of 0 or 1, and with a life expectancy of at least six months. They all had histologically confirmed prostate cancer with no evidence of small cell or neuroendocrine differentiation. Their metastatic disease had to be clinically or radiologically documented. All patients received prior hormonal treatment with at least one of either abiraterone, enzalutamide, apalutamide, or other next-generation androgen receptor pathway inhibitors. Patients were allowed to receive cytotoxic therapy when they were in the castrate sensitive setting and also up to one regimen of cytotoxic therapy during their castrate-resistant setting.
The primary objective of this study was to determine the clinical benefit rate for each treatment arm, defined as the proportion of patients with PSA decline of 50% or above, or measurable disease, complete or partial response or stable disease for 12 weeks or more. Patient disposition is shown in Table 1, and baseline demographic data are shown in Table 2.
Table 1 – Patient disposition:
Table 2 – Baseline patient demographic data:

The PSA declines, ctDNA genomic landscapes and ctDNA profiles for patients enrolled on the Adavosertib and Darolutamide arms are shown in Figure 2, Table 3, and Figure 3, respectively.
Figure 2 – PSA declines: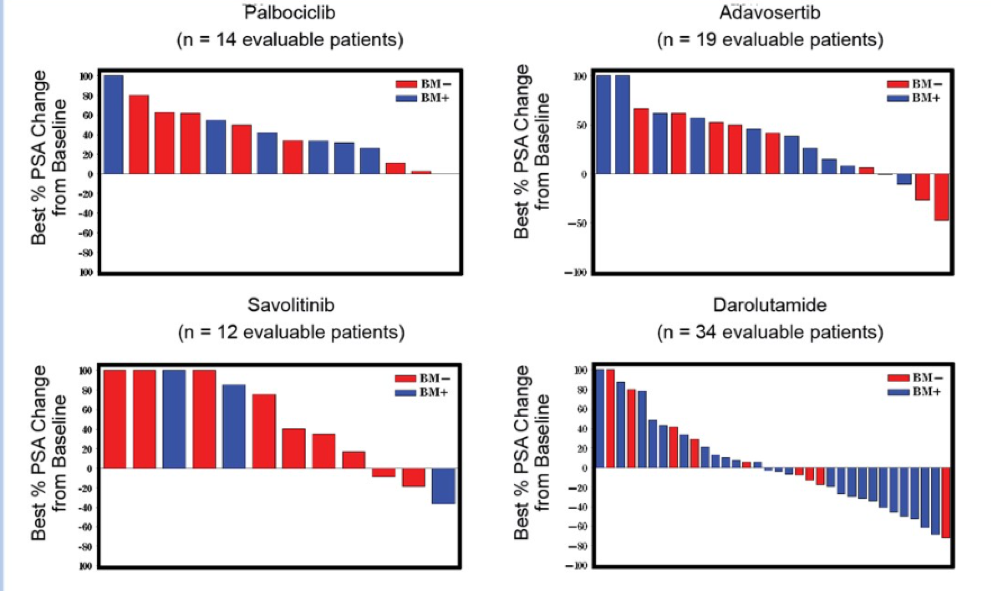
Table 3 – ctDNA genomic landscapes:
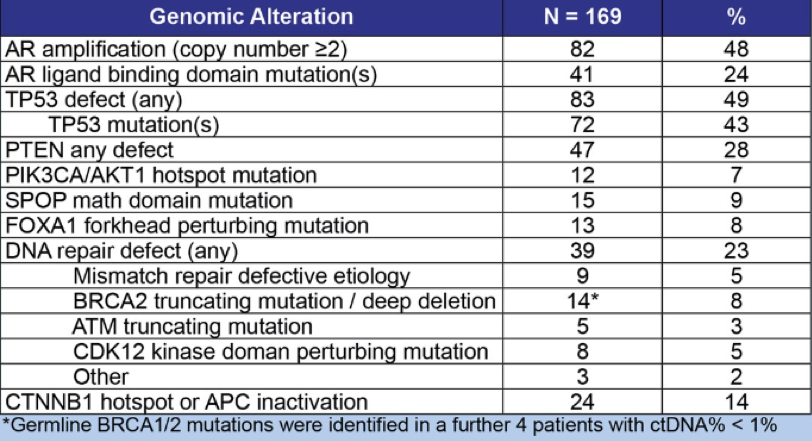
Figure 3 – ctDNA profiles for patients enrolled in the Adavosertib and Darolutamide arms:

The authors concluded that this analysis demonstrates that prospective centralized screening of ctDNA for genomic stratification of MCRPC patients into a precision oncology trial is feasible. The authors demonstrated activity in four out of the seven evaluable cohorts with darolutamide and adavosertib, which met the criteria and threshold for expansion of these arms. Lastly, this PC-BETS umbrella study has been able to establish an efficient platform to evaluate novel targeted therapies in patients with metastatic castrate-resistant prostate cancer. This study continues to accrue patients, and according to the authors, additional treatment arms will be added.
Presented by: Kim N Chi, MD, FRCP(C), BC Cancer, and Vancouver Prostate Cancer Center, Vancouver, BC, Canada
Written by: Hanan Goldberg, MD, MSc., Urology Department, SUNY Upstate Medical University, Syracuse, NY, USA, @GoldbergHanan, at the 2020 ASCO Annual Meeting, Virtual Scientific Program #ASCO20, May 29-31, 2020.


