Radium 223 is a radiopharmaceutical with structural similarity to calcium, which enables it to be absorbed by the bone where it emits alpha particles, and therefore could have utility in the treatment of micrometastatic bone disease.
The primary goal of this planned phase 2 RAVENS trial is to evaluate the efficacy of stereotactic ablative radiation (SABR) with or without radium 223 in prolonging progression-free survival in men with hormone-sensitive oligometastatic prostate cancer. The primary endpoint of this study is to assess whether SABR with radium 223 will increase median progression-free survival from 10 to 20 months.
The inclusion criteria include only men with hormone-sensitive oligometastatic prostate cancer with 3 or less metastasis with at least 1 bone metastasis, shown by conventional imaging. The PSA doubling time has to be less than 15 months, and patients have to have an ECOG performance status of <=2. Patients who have castrate levels of testosterone or are treated with androgen deprivation therapy will be excluded.
In this study, patients will be randomized using the ratio of 1:1 to either SABR or SABR+ radium 223 (6 doses every 4 weeks).
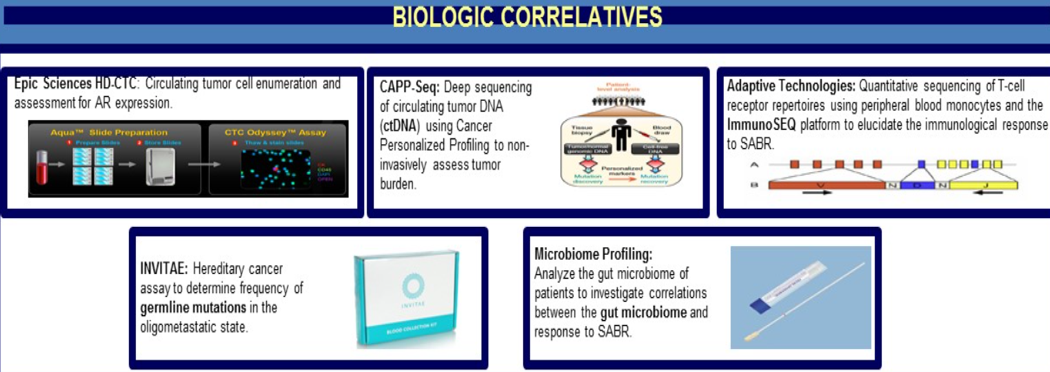
The authors also plan to measure changes in circulating tumor cells, deep sequencing of circulating tumor DNA, and T cell repertoire profiling.
The details of the study design are shown in Figure 1, and the details of the biologic correlatives that will be collected in this study are elaborated in Figure 2.
Figure 1 – Study design:
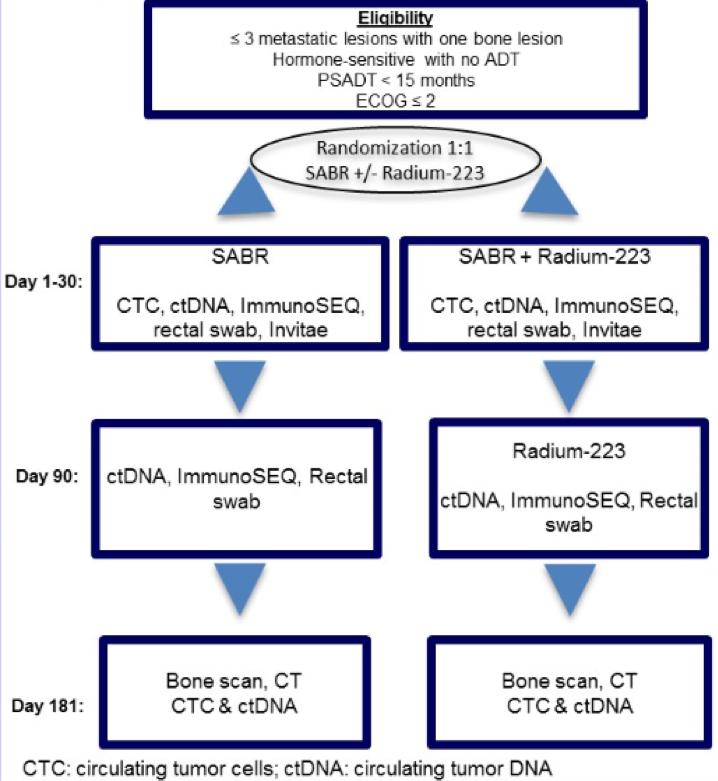
Figure 2 – Biologic correlatives:
Presented by: Matthew Pierre Deek, MD, Johns Hopkins School of Medicine
Written by: Hanan Goldberg, MD, MSc., Urology Department, SUNY Upstate Medical University, Syracuse, NY, USA @GoldbergHanan at the 2020 ASCO Annual Meeting, Virtual Scientific Program #ASCO20, May 29-31, 2020.
Related Content:
Clinical Trial Information: NCT04037358


