(UroToday.com) There has been a rapid evolution in treatment options for patients with non-metastatic castration-resistant prostate cancer since the spring of 2018. Up until the presentation of SPARTAN and PROSPER trials, reporting on the use of apalutamide and enzalutamide in non-metastatic castration-resistant prostate cancer, at GU ASCO in February 2018, there were no specifically approved treatment options for these patients. These agents, as well as darolutamide, were subsequently approved based on demonstrated improvements in metastasis-free survival. While metastasis-free survival is a reasonable surrogate for overall survival in this population, there were questions as to whether the benefit of using androgen-axis inhibitors would translate to overall survival benefits, in part due to the differential survival effect seen according to disease burden in CHAARTED. While overall survival data from SPARTAN have been previously reported, at the time of initial presentation and subsequent publication, overall survival data from PROSPER were immature with only 165 of 596 (28%) prespecified deaths.
During the Poster Discussion section of the virtual 2020 ASCO Annual Meeting, Cora Sternberg, MD, and colleagues presented the overall survival data assessing enzalutamide in non-metastatic castration-resistant prostate cancer from PROSPER.
The methodology of the PROSPER trial has previously been reported and published. In brief, men with non-metastatic castration-resistant prostate cancer, PSA doubling time ≤ 10 mo, and PSA ≥ 2 ng/mL at screening were randomized in a 2:1 fashion to enzalutamide 160mg or placebo, in addition to continuing androgen deprivation therapy.
Given the primary outcome, as previously reported, of metastasis-free survival, overall survival was assessed as a key secondary outcome utilizing a group sequential testing procedure with O’Brien-Fleming-type alpha spending function. As a result of this approach, to be considered statistically significant, a p-value of 0.021 was required. Median overall survival was assessed using the Kaplan Meier technique; hazard ratios and associated 95% confidence intervals were calculated using a stratified Cox regression model.
With a data cut off of October 15, 2019, the median follow-up was approximately 48 months. There were a total of 466 deaths (288 [30.9%] and 178 [38.0%] in the enzalutamide and placebo arms, respectively).
Median overall survival was 67.0 months (95% CI 64.0-not reached) among patients receiving enzalutamide and 56.3 months (95% CI 54.4-63.0) among patients receiving placebo, corresponding to a 27% relative reduction in the risk of death for patients receiving enzalutamide (hazard ratio 0.73; 95% confidence interval 0.61 to 0.89; P = .0011).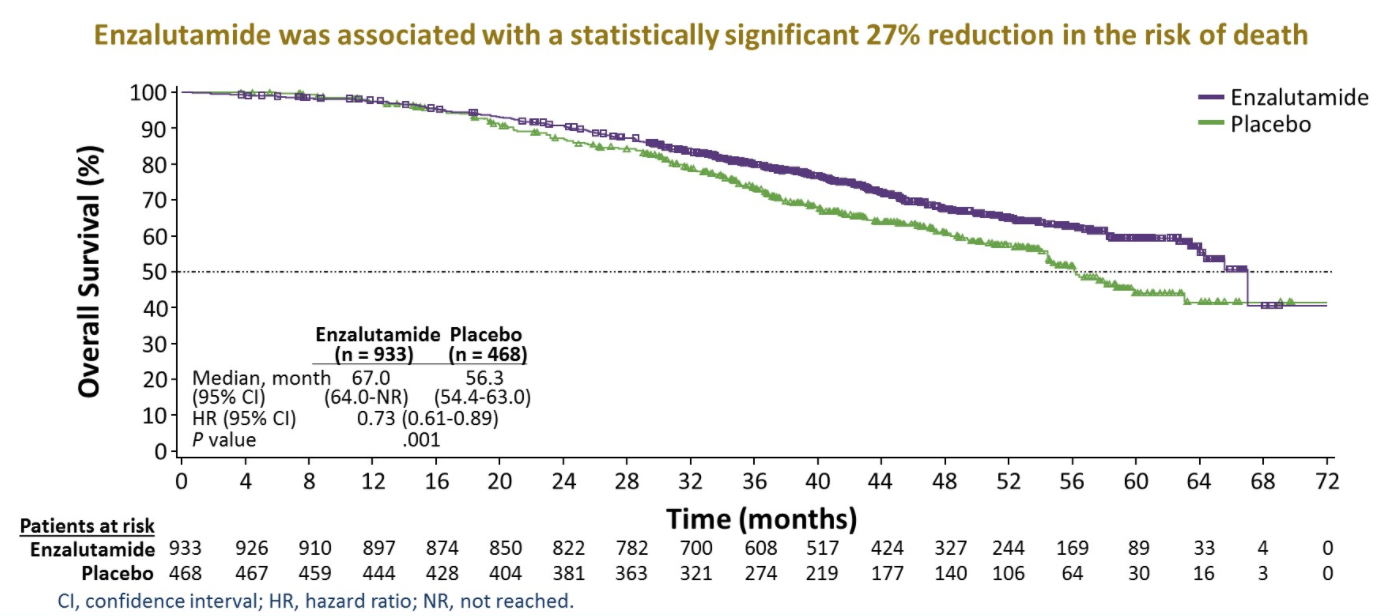
Subsequent anti-neoplastic therapies, including androgen axis inhibitors and cytotoxic chemotherapy, were administered in 310 (33%) of patients in the enzalutamide arm and 303 (65%) of patients in the placebo arm following study treatment discontinuation.
As may be expected from the decreased risk of metastatic progression previously reported from PROSPER, patients receiving enzalutamide had a much longer duration of treatment, compared to those receiving placebo (33.9 months vs. 14.2 months).
Toxicity was similar to the initial report of the PROSPER trial with Grade ≥ 3 adverse events (AEs) reported by 48% of men in the enzalutamide arm vs 27% in the placebo arm (16% vs 6% were drug-related, respectively). Falls, fatigue, and hypertension were the adverse events with rates >2 events per 100 patient-years higher among treated patients.
In conclusion, an updated report of the PROSPER trial demonstrates a significant improvement in overall survival for men receiving enzalutamide for non-metastatic castration-resistant prostate cancer, despite higher utilization of subsequent antineoplastic therapies and cross-over.
Presented by: Cora N. Sternberg, MD, FACP, Clinical Director of the Israel Englander Institute for Precision Medicine, Weill Cornell Medicine, Former Chief of the Department of Medical Oncology at the San Camillo-Forlanini Hospital in Rome, Italy
Co-Authors: Cora N. Sternberg, Karim Fizazi, Fred Saad, Neal D. Shore, Ugo De Giorgi, David F. Penson, Ubirajara Ferreira, Petro Ivashchenko, Eleni Efstathiou, Katarzyna Madziarska, Michael Paul Kolinsky, Daniel Iracema Cubero, Bettina Noerby, Fabian Zohren, Xun Lin, Katharina Modelska, Jennifer Sugg, Joyce Leta Steinberg, Maha H. A. Hussain
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center, Nashville, Tennessee, Twitter: @WallisCJD, at the 2020 ASCO Annual Meeting, Virtual Scientific Program #ASCO20, May 29-31, 2020.
Related Content:
Read: Enzalutamide and Survival in Nonmetastatic, Castration-Resistant Prostate Cancer
View: The PROSPER Trial: Chemotherapy-Related endpoints in Patients with nmCPRC - Neal Shore


