(UroToday.com) There are currently significant imaging deficiencies for men with prostate cancer (Figure 1), with the hope that PSMA imaging-based modalities will address a significant gap in this field. Prostate-specific membrane antigen PSMA is a transmembrane glycoprotein with folate hydrolase activity. It is very specific in differentiating benign and malignant prostate cells. It has been shown to be overexpressed in prostate cancer cells, and especially in metastatic and castrate-resistant disease.
Prostate-specific membrane antigen (PSMA) is expressed 100 to 1,000 times more highly in prostatic adenocarcinoma than in benign disease. Globally, there is a significant, rapid adoption of PSMA PET-CT/MRI, which can detect metastatic disease that is inapparent on conventional imaging (CT and bone scintigraphy). PSMA is the single most well-established prostate restricted cell membrane target that is known to man. It has been validated in both cells and in humans in the clinic, and it is distributed mostly to the prostate and in prostate cancer. PSMA remains a target of high interest in the current era, and it is especially important for higher grade, advanced, metastatic tumors that continue to grow despite hormonal therapy.
Figure 1: Imaging Deficiencies for Men with Prostate Cancer

PSMA is a ligand that is characterized by being a small molecule with a short circulation time (hours), meaning that the optimal tumor imaging is within hours after its administration, and it can rapidly diffuse to all sites of expression. This contrasts with the monoclonal antibody, which is a large molecule with long circulation time (days), giving it an ability to optimally used for tumor imaging at three to 8 days following administration. It is able to target mostly by the vasculature. There is a third category of mini bodies, which are the intermediate category located between the small ligand molecules and the large monoclonal antibodies.
In work presented by Dr. Thomas Hope in abstract 5502, 633 men with intermediate or high-risk prostate cancer considered for surgery were analyzed. 277 men eventually had a prostatectomy. The study had excellent inter-reader correlation, but the imaging results probably influenced the treatment. Interestingly, not all positive lymph nodes were captured with surgery.
In the CONDOR study, presented by Dr. Morris et al. at ASCO 2020, The authors assessed the role of 18F-DCFPyL for prostate cancer biochemical recurrence. In this study, the authors compared the current localization rate to the composite standard of truth. They used baseline imaging, which included mostly CT scans and bone scans. This study’s results are summarized in Figure 1.
Figure 1 – CONDOR study:
In the OSPREY study presented in ASCO 2020, the authors assessed the diagnostic performance of PyL PET/CT for pelvic lymph nodes in high-risk prostate cancer disease.
Scott T. Tagawa, MD, MS, FACP continued his talk and stated that the standard of care is dynamically changing. We are still learning what agents are available and at what stage we can use them. We need to learn if there are clinically significant differences between the various agents or if we should pick which agent to use based upon availability or cost factors. We must also need to consider the reader learning curve in the beginning. Current limitations include a lack of large studies with serial imaging. Therefore, we cannot yet replace CT, MRI, and bone scans with these modalities. Lastly, it is important for us to understand how these new imaging modalities will influence long-term outcomes.
The TheraP study1 was a randomized phase 2 trial of 177 Lu-PSMA-617 theranostic treatment vs. cabazitaxel in progressive metastatic castration-resistant prostate cancer (Clinical Trial Protocol ANZUP 1603). Patients with metastatic castrate-resistant prostate cancer who posted docetaxel therapy and suitable for cabazitaxel were enrolled. All patients had progressive disease with rising PSA above 20 ng/ml. All patients had adequate renal, hematologic, and liver function with an ECOG performance status of between 0-2. All patients underwent PSMA PET and FDG PET. The primary endpoint was the rate of over 50% PSA decline with no known PSA expression effect by targeting PSMA. Sensitivity analysis of per-protocol treated consistent with the intention to treat analysis, demonstrating a 23% absolute difference for PSA response with a hazard ratio of 0.67 for PSA progression-free survival. The results of radiographic progression-free survival and overall survival analysis are still awaited.
The VISION Study (Figure 2) will put the results of the TheraP study into a clinical context. It will randomize metastatic castrate-resistant prostate cancer patients to either the best standard of care with Lu-PSMA-617 treatment or only to the best standard of care.
Figure 2 – VISION study design:
Dr. Tagawa continued with some future predictions regarding PSMA usage in prostate cancer in the near future:
- PSMA modalities will be used in earlier stages of the disease which are perhaps less radioresistant and harbor less PSMA heterogeneity
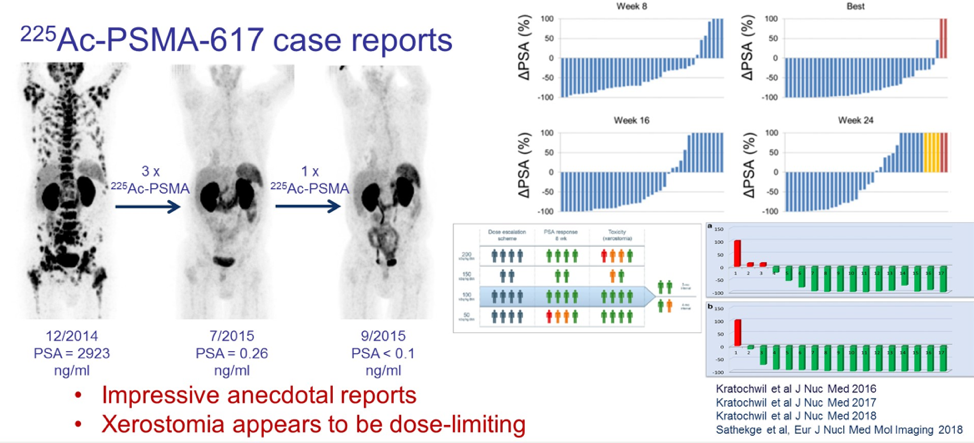
- PSMA targeted alphas will be used as well, which is currently an experimental modality (Figure 3).
- Multiple rational combinations will be more frequently tested. These include lutetium and docetaxel, lutetium-J591 + lutetium-PSMA-617, lutetium-PSMA+ pembrolizumb, and lutetium-PSMA + Olaparib.
Figure 3 – PSMA-targeted alphas:
Dr. Tagawa concluded his talk and emphasized that in this virtual ASCO 2020 meeting, we have seen translational advances lead to real, clinically relevant improvements with a high likelihood for broader availability in the near term.
Presented by: Scott T. Tagawa, MD, MS, FACP, Professor of Medicine and Urology at Weill Cornell Medicine, Attending Physician at New York-Presbyterian, NY
Written by: Hanan Goldberg, MD, MSc., Urology Department, SUNY Upstate Medical University, Syracuse, NY, USA, Twitter: @GoldbergHanan, at the 2020 ASCO Annual Meeting, Virtual Scientific Program #ASCO20, May 29-31, 2020.
References:
- Hofman MS, Emmett L, Violet J, et al. TheraP: a randomized phase 2 trial of (177) Lu-PSMA-617 theranostic treatment vs cabazitaxel in progressive metastatic castration-resistant prostate cancer (Clinical Trial Protocol ANZUP 1603). BJU international 2019; 124 Suppl 1: 5-13.


