TALAPRO-1 is enrolling patients (~100) with measurable soft tissue disease, progressive mCRPC, and DDR mutations likely to sensitize to PARP inhibition (ATM, ATR, BRCA1/2, CHEK2, FANCA, MLH1, MRE11A, NBN, PALB2, RAD51C). These patients must have received 1–2 chemotherapy regimens (≥1 taxane-based) for metastatic disease and progressed on ≥1 novel hormonal therapy (enzalutamide/abiraterone acetate) given for mCRPC. DDR mutations are defined as known/likely pathogenic variants or homozygous deletions. Patients receive oral talazoparib 1 mg/day (moderate renal impairment 0.75 mg/day) until radiographic progression, unacceptable toxicity, consent withdrawal or death. The primary endpoint for this study is objective response rate (ORR), and secondary endpoints include time to OR, response duration, PSA decrease ≥50%, circulating tumor cell (CTC) count conversion (to CTC = 0 and <5 per 7.5 mL blood), time to PSA progression, radiographic PFS, overall survival, and safety. Soft tissue responses must be confirmed >=4 weeks later by CT or MRI with no evidence of confirmed bone progression per PCWG3 criteria on repeat bone scan >= 6 weeks later. A planned efficacy/safety interim analysis was done when 60 patients with DDR mutations and measurable disease completed ≥6 months of talazoparib.
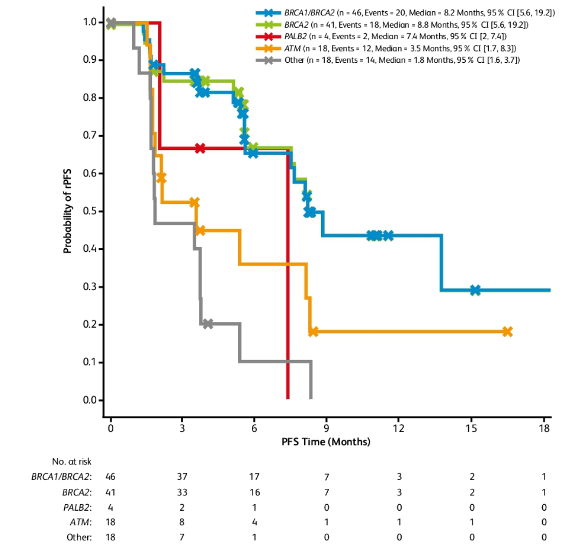
There were 113 patients that received talazoparib at the time of data cutoff on December 12, 2019. Among these patients, 60.2% had discontinued treatment: 41 for disease progression, six for adverse events, four secondary to death, three for global deterioration of health status, one secondary to no longer achieving a clinical benefit and 13 for other reasons. The median duration of treatment was 4.5 months (range 0.1-21.1) months in the safety population and 4.8 months (range 0.1-21.1) months in the DDR-deficient measurable disease population. There were 86 patients among the DDR-deficient population who were evaluable for efficacy response, of which all patients had received prior docetaxel therapy and 38 patients (44.2%) had received prior cabazitaxel. Overall ORR was 26.7% of which a confirmed ORR of 41.5% was found in patients with BRCA1/2 alterations, and 40.5% for patients with BRCA2 alterations. In patients with BRCA1/2 alterations, the median rPFS was 8.2 months (95% CI 5.6-19.2) and median rPFS was longer in BRCA2 alterations (8.8 months, 95% CI 5.6-19.2) compared to patients with PALB2, ATM, and other DDR alterations (range: 1.8-7.4 months):
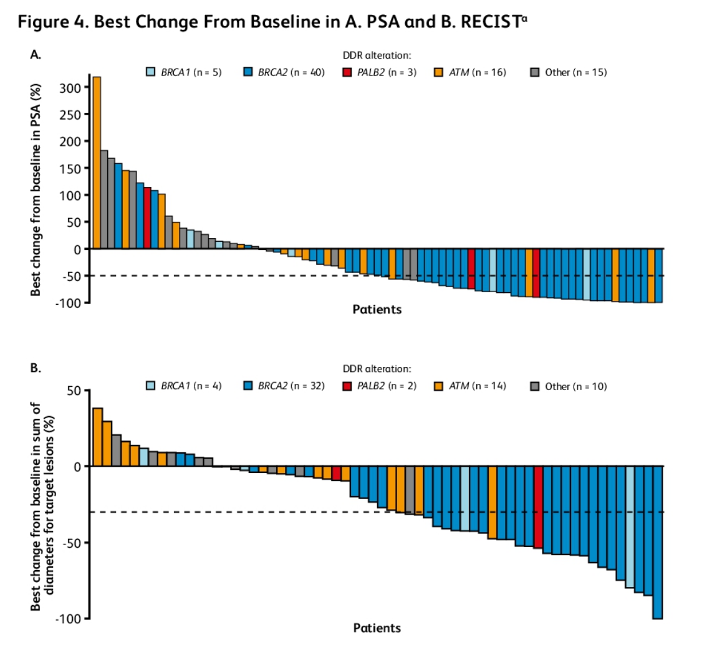
Additionally, best change in PSA level and sum of diameters for target lesions were assessed in 79 and 62 patients from the DDR-deficient population, respectively:
The most common treatment-emergent adverse events were anemia (42.5%) and nausea (32.7%).
In this interim analysis of TALAPRO-1, talazoparib monotherapy demonstrated antitumor activity in mCRPC patients with DDR alterations who have previously received taxane therapy and novel hormonal therapy with confirmed ORR of 26.7%. Efficacy was most notable for a subset of patients with mCRPC who tumors had BRCA1/2 alterations, who had a confirmed ORR of 41.5%. Finally, talazoparib monotherapy was generally well tolerated with no new safety signals of concern.
Presented by: Johann S. De Bono, MB, ChB, FRCP, MSc, PhD, FMedSci, The Royal Marsden Hospital and The Institute of Cancer Research, London, United Kingdom
Co-Authors: Niven Mehra, Celestia S. Higano, Fred Saad, Consuelo Buttigliero, Inge M. van Oort, Marielena Mata, Hsiang-Chun Chen, Cynthia G. Healy, M. Luisa Paccagnella, Akos Czibere, Karim Fizazi; The Royal Marsden Hospital and The Institute of Cancer Research, London, United Kingdom; Department of Oncology, Radboud University Medical Center, Nijmegen, Netherlands; Fred Hutchinson Cancer Research Center, Seattle, WA; Centre Hospitalier de l’Université de Montréal (CHUM), Montréal, QC, Canada; Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Orbassano, Turin, Italy; Department of Urology, Radboud University Medical Center, Nijmegen, Netherlands; Pfizer Inc., San Diego, CA; Pfizer Inc., Collegeville, PA; Pfizer Inc., Groton, CT; Pfizer Inc., Cambridge, MA; Institut Gustave Roussy, University of Paris Sud, Villejuif, France
Written By: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the 2020 ASCO Annual Meeting, Virtual Scientific Program #ASCO20, May 29- 31, 2020
References:
1. de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020.
Related Content:
Read: ASCO GU 2020: A Phase II Study of Talazoparib in Men with DNA Damage Repair Mutations and Metastatic Castration-Resistant Prostate Cancer


