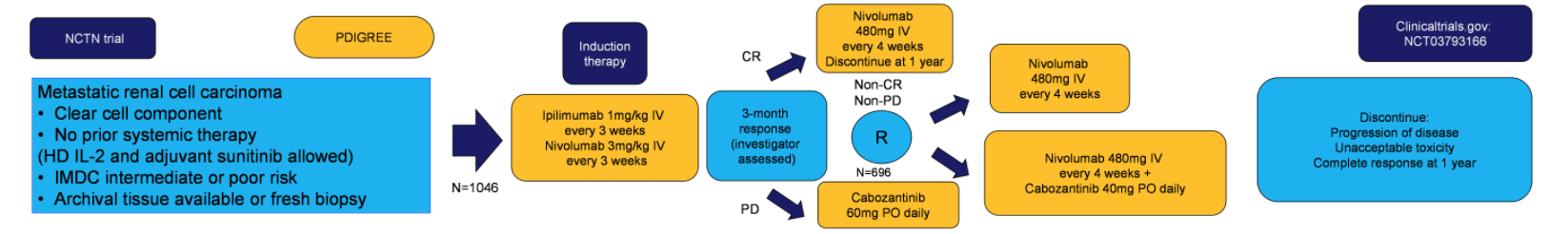
This study, led by the Alliance Cooperative Group and open across all NCTN centers, has currently enrolled 130 patients and is planning to enroll a total of 1046 patients. Patients will receive induction therapy per the CheckMate-214 protocol of ipilimumab 1mg/mg + nivolumab 3 mg/kg every three weeks for a total of four cycles. Following this induction period, the investigator will assess the treatment response to assign the patient into one of three treatment groups. For patients with progressive disease, patients will switch to cabozantinib 60 mg daily. For patients with a complete response, they will proceed with maintenance nivolumab. For patients with a partial response or stable disease, patients will be randomly assigned to either maintenance nivolumab or nivolumab in combination with cabozantinib 40 mg daily. Preliminary data shows that this combination of cabozantinib/nivolumab is clinically active not only in renal cell carcinoma (RCC) (CheckMate 9ER) but also in urothelial carcinoma1 and recurrent endometrial cancer.2 The primary objective is three-year overall survival for the randomized cohorts of nivolumab vs nivolumab+cabozantinib. Secondary objectives are shown below: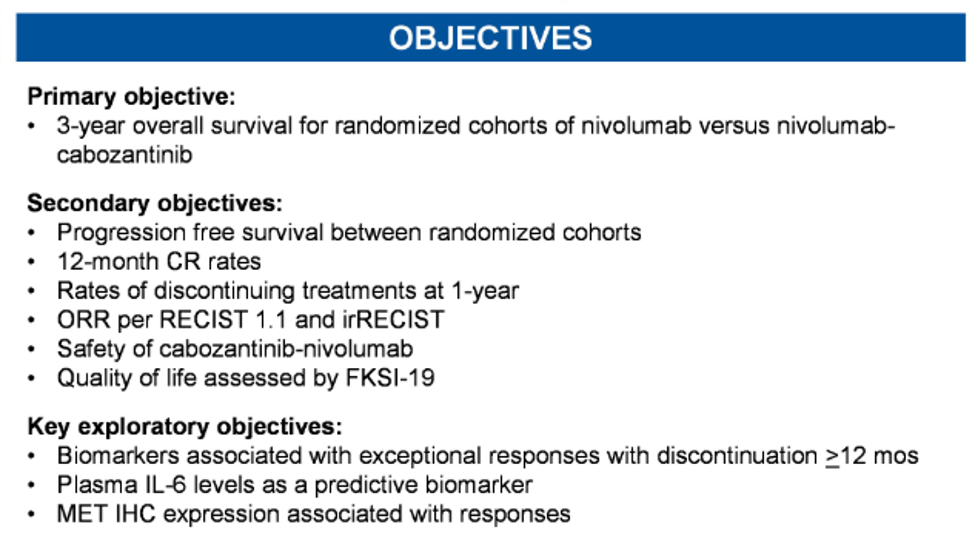
PDIGREE is a novel adaptive frontline immunotherapy study which upon completion will help answer many important questions regarding treatment duration, when to combine VEGF therapy with IO therapy, and which patients benefit most from IO-VEGF combination upfront vs. sequential therapy. This trial is actively enrolling patients at all National Clinical Trials Network (NCTN) study sites.
Presented by: Tian Zhang, MD, Assistant Professor of Medicine, Member of the Duke Cancer Institute, Department of Medicine, Duke University School of Medicine, Chapel Hill, North Carolina
Written by: Jason Zhu, MD, Medical Oncologist, Division of Genitourinary Cancers, Levine Cancer Institute, Charlotte, North Carolina, Twitter: @TheRealJasonZhu, at the 2020 ASCO Annual Meeting, Virtual Scientific Program #ASCO20, May 29-31, 2020.
- da Motta Girardi, Daniel, Scot Anthony Niglio, Amir Mortazavi, Primo Lara, Sumanta K. Pal, Biren Saraiya, Lisa M. Cordes et al. "Phase I expansion study of cabozantinib plus nivolumab (CaboNivo) in metastatic urothelial carcinoma (mUC) patients (pts) with progressive disease following immune checkpoint inhibitor (ICI) therapy." (2020).
- Lheureux, Stephanie, Daniela Matei, Panagiotis A. Konstantinopoulos, Matthew Stephen Block, Andrea Jewell, Stephanie Gaillard, Michael S. McHale et al. "A randomized phase II study of cabozantinib and nivolumab versus nivolumab in recurrent endometrial cancer." (2020).


