The first abstract Dr. Sridhar discussed was Abstract 5017 “Final results of PEANUT – Pembrolizumab and nanoparticle albumin-bound paclitaxel (nab-paclitaxel) as salvage therapy for metastatic urothelial carcinoma”. Dr. Sridhar started by highlighting that metastatic urothelial carcinoma is an aggressive, incurable disease. First-line treatment remains platinum-based chemotherapy, with high response rates, but rarely durable. Until recently, taxanes were the mainstay of treatment, however, immune checkpoint inhibitors are the new standard of care. The landscape of metastatic urothelial carcinoma has changed significantly over the last five years, with five immune checkpoint inhibitors approved in the platinum-refractory setting. The KEYNOTE-045 trial lead to the approval of pembrolizumab in the platinum-refractory setting, showing a survival improvement for pembrolizumab of 3 months with an HR of 0.73 (95% CI 0.59-0.91).1 Importantly, these results were maintained over two years of follow-up. Looking closely at the five approved immune checkpoint inhibitors in the platinum-refractory setting, Dr. Sridhar notes that response rates are only between 13-20%, thus the majority do not respond – new therapies are urgently needed for these patients. Paclitaxel has previously shown response rates of 22-25% in the platinum-refractory metastatic urothelial carcinoma setting, is inexpensive and is generally well-tolerated. So, the question is: can immune checkpoint inhibitors and taxanes be safely combined for patients with metastatic urothelial carcinoma?
The study design for PEANUT is as follows:
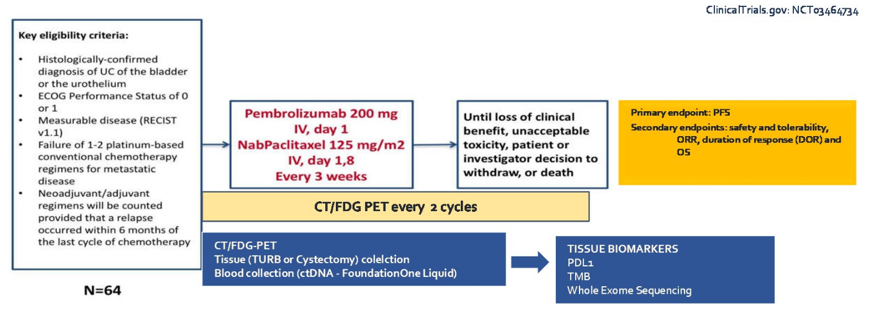
The primary endpoint for this study was progression free survival, incorporating an FDG-PET into the follow-up imaging regimen. Of note, patients enrolled in this study had an ECOG performance status of 0-1, as opposed to 0-2 seen in many trials in this setting. Dr. Sridhar notes that it is important moving forward to harmonize eligibility criteria across trials and the incorporate health-related quality of life metric assessments in this metastatic urothelial carcinoma setting. Looking at the key baseline characteristics, Dr. Sridhar highlights that enrollment was rapid for this trial, with 70 patients participating at a single site from January 2019 to February 2020. Most patients were ECOG 0, had lymph node only disease, and a Bellmunt risk score of 0. All cause adverse events in PEANUT was 33%, which was lower than for pembrolizumab in KEYNOTE-045 (any grade – 61%; grade 3-4 – 15%). This may be secondary to the healthier treatment population, according to Dr. Sridhar. The objective response rate (ORR) for PEANUT was 38.6% and PFS was 5 months. Patients that had PDL1 >10%, lymph node only disease and Bellmunt Risk score of 0 had improved efficacy results, which is encouraging. Furthermore, treatment is ongoing in 29 of the 70 patients in this trial. Dr. Sridhar notes that this highlights that metastatic urothelial carcinoma is a chemosensitive disease and the PFS improvement seen in PEANUT is similar to other studies of combination approaches. Also in this space is enfortumab, erdafitinib, and sacituzumab, all of which are currently being tested in the phase 3 setting.
Key questions looking ahead from PEANUT according to Dr. Sridhar are as follows:
- Are there populations that may benefit from the PEANUT regimen? These may include patients that have progressed rapidly post-perioperative chemotherapy and patients without access to the newer agents
- If immune checkpoint inhibitors move into the front line setting in combination or as a switch maintenance, what are the downstream implications? Perhaps it will be feasible to rechallenge patients with immune checkpoint inhibitors, but it remains to be delineated how we will optimally sequence these new drugs
- There is a need for biomarkers for both response and toxicity to help guide us to make the right choice for the right patient at the right time
The final abstract discussed was Abstract 5018 “Phase II study of nivolumab and ipilimumab for advanced rare genitourinary cancers”. Dr. Sridhar notes that rare cancers represent about 25% of all cancers and are defined as affecting less than 40,000 people annually. There are major challenges for these patients, including delays in diagnosis, lack of expertise, understanding coming from case reports and small case series, heterogeneity within small groups of patients, lack of industry interest for support, and low prevalence making it challenging to accrue to clinical trials. Thus, collaboration is key to these trials. For the trial presented by McGregor and colleagues, eligible patients had a metastatic rare genitourinary malignancy, ECOG performance status of 0-2, and no prior immune checkpoint inhibitor exposure. Eligible patients received nivolumab 3 mg/kg and ipilimumab 1 mg/kg intravenously every 3 weeks for 4 doses with continued nivolumab 480 mg IV every 4 weeks. The primary endpoint was overall response rate (ORR) by RECIST 1.1. There were 57 patients enrolled at six institutions in 3 cohorts: bladder cancer of variant histologies (n = 19), adrenal cortical carcinoma (n = 19), and other tumors (n = 19). Dr. Sridhar notes that in rare populations it is important to consider trial designs that aim to reduce barriers and maximize accrual, which the trialists for this trial have done. Non-urothelial bladder cancers include squamous, neuroendocrine, and adenocarcinoma, amongst other rare variants. Typically, these are aggressive and may present with non-specific/atypical symptoms and currently, there are no clear guidelines for management. Importantly, most of these patients are excluded from metastatic urothelial carcinoma clinical trials. In the SAUL trial testing atezolizumab,2 there were 47 non-urothelial patients included, with an ORR of 9%, median PFS of 2.1 months, and median OS of 7.3 months. In the current trial, patients with bladder cancer of variant histologies had an ORR of 37% (80% CI 22-54%), median PFS of 9.2 months, and 12-month OS rate of 66% (95% CI 40-83%). Dr. Sridhar notes that this is encouraging data in the non-urothelial setting, with better results noted than in previous studies. Looking ahead, Dr. Sridhar notes that this trial adds important information about rare genitourinary tumors and correlative studies may prove to be quite informative. Ultimately, it is great to see interest and trials in these rare tumors.
Presented by: Srikala Sridhar MD, MSc, FRCPC, Associate Professor, Department of Medicine, Division of Medical Oncology at the University of Toronto.
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the 2020 ASCO Annual Meeting, Virtual Scientific Program #ASCO20, May 29-31, 2020.
References:
- Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med 2017;376(11):1015-1026.
- Sternberg CN, Loriot Y, James N, et al. Primary results from SAUL, a multinational single-arm safety study of atezolizumab therapy for locally advanced or metastatic urothelial or nonurothelial carcinoma of the urinary tract. Eur Urol 2019 Jul;76(1):73-81.


