This study is a multicenter, prospective phase II trial of multimodal therapy in patients with localized urothelial carcinoma of the bladder in clinical stages T2-4a N0 M0, ECOG 0-1, without contraindications to immunotherapy, who either wish for bladder preservation or are ineligible for cystectomy. The treatment consists of initial transurethral resection of the tumor, followed by durvalumab 1500 mg IV plus tremelimumab 75 mg IV, every 4 weeks for three doses. Normofractionated external-beam radiotherapy is started two weeks later, at doses of 46 Gy to the minor pelvis and 64-66 Gy to the bladder. The trial design is depicted in the following figure:
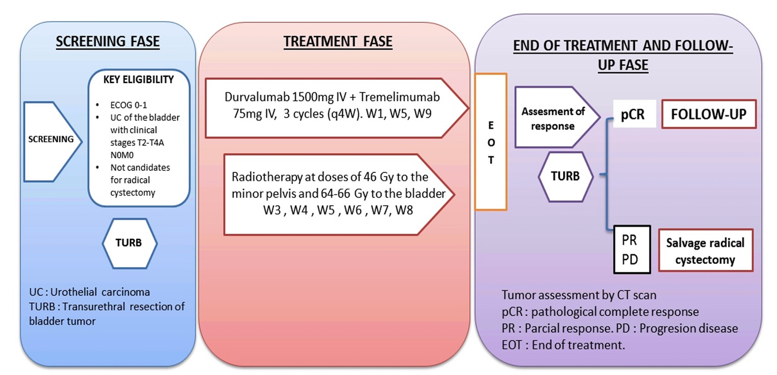
The primary endpoint is pathological response (≤T1) at post-treatment biopsy. Secondary outcomes include:
- Rate of patients with bladder preservation
- Rate of immediate and late salvage cystectomies
- Survival with bladder preserved free of tumor
- Disease free survival
- Overall survival
- Long-term functionalism and late sequelae of treatment among those with preserved bladders
- Safety and tolerability
Exploratory objectives are to analyze the changes in the tumor cell and inflammatory stroma induced by the study treatment in patients with poor response and to analyze the predictive value of immune biomarkers on response and bladder preservation. A 2-stage sequential design (response rate P0=5, P1=0.7, α=0.10, β=0.20) requires at least six responses in the first 12 patients to expand to a second cohort of 20 patients. Patients with pathological response will be candidates to bladder preservation, whereas those with residual muscle invasive tumors will be candidates to salvage cystectomy. At the present time, the prespecified activity goal for the first stage of accrual was met and the second stage accrual began in December 2019.
Clinical trial information: NCT03702179.
Presented by: M. Andres Cuellar, MD, Catalan Institute of Oncology, Barcelona, Spain
Co-Authors: Ana Medina, Regina Girones, B.P. Valderrama, Albert Font, MJ Juan-fita, Guillermo de Velasco, Ferran Ferrer, Francesc Vigués, Xavier Garcia del Muro; Catalan Institute of Oncology, Barcelona, Spain; Centro Oncologico de Galicia, A Coruña, Spain; Hospital Universitario La Fé, València, Spain; Department of Medical Oncology, Hospital Universitario Virgen del Rocío, Seville, Spain; Institut Català d'Oncologia, Hospital Germans Trias i Pujol, Barcelona, Spain; Fundación Instituto Valenciano de Oncología, Valencia, Spain; Medical Oncology Department, Hospital Universitario 12 de Octubre, Madrid, Spain; Instituto Catalan Oncologia, Barcelona, Spain; Hospital Universitario de Bellvitge, Barcelona, Spain; Catalan Institute of Oncology, IDIBELL, University of Barcelona, Barcelona, Spain
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md
References:
- James ND, Hussain SA, Hall E, et al. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med 2012 Apr 19;366(16):1477-1488.
- Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015 Apr 16;520(7547):373-377.


