This abstract describes the outcomes of 98 chemo-naive patients with metastatic castration-resistant prostate cancer (mCRPC) treated with enzalutamide. Tumor samples were analyzed using polymerase chain reaction (PCR), fluorescence in situ hybridization (FISH), and immunohistochemistry (IHC) for ERG, and exploratory biomarkers also included plasma DNA, AR copy number by ddPCR and CTC by AdnaTest. After a median follow up of 37.3 months, 82% of patients achieved a PSA50 and the median prostate-specific antigen (PSA) progression-free survival (PFS) was 13.7 months, similar to PREVAIL (78% of patients achieved PSA50, median PSA PFS 11.2 months).5
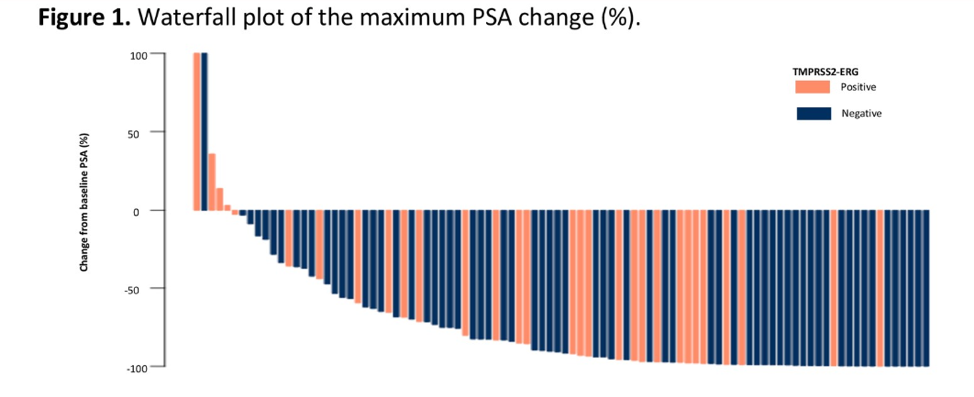
33% of patients had a TMPRSS2-ERG fusion. There was no difference in PSA response or PSA PFS between patients with the gene fusion and those without.
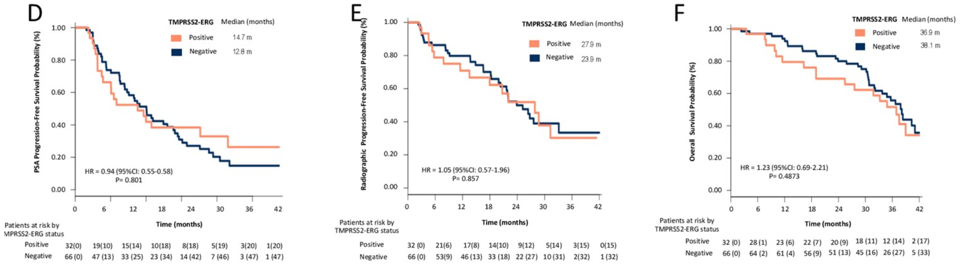
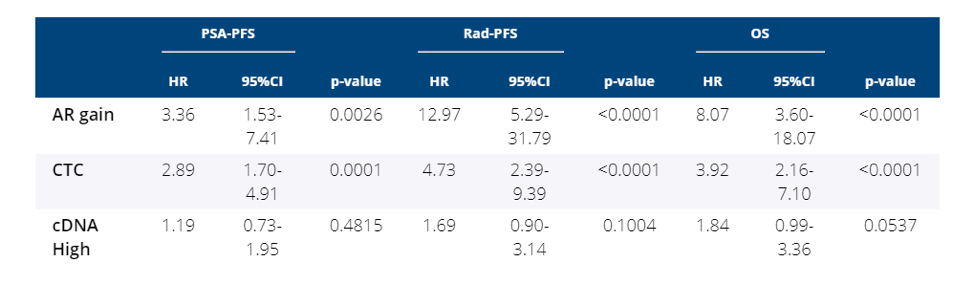
Plasma AR gain was associated with worse PSA-PFS, radiologic PFS, and median overall survival.
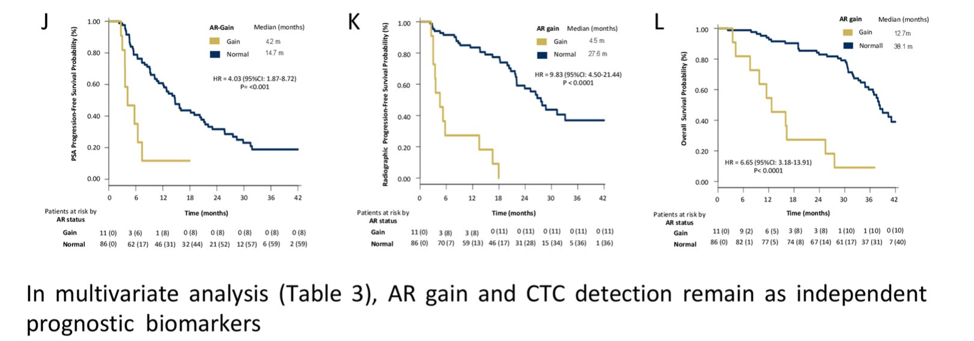
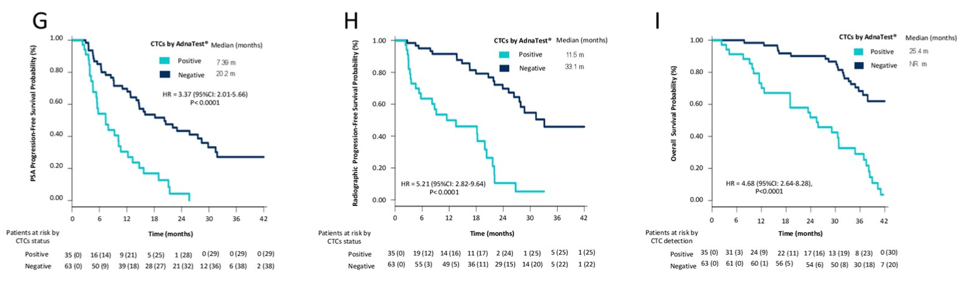
In this prospective analysis of chemo-naive patients with metastatic castration-resistant prostate cancer (mCRPC) treated with enzalutamide, the TMPRSS2-ERG gene fusion does not predict sensitivity or resistance to enzalutamide. However, plasma AR gain and detection of CTCs were strongly predictive of adverse outcomes, including decreased PSA PFS, radiologic PFS, and overall survival.
Presented by: Enrique Grande, MD, PhD, MD Anderson Cancer Center Madrid, Madrid, Spain
Written by: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University, @TheRealJasonZhu at the 2019 ASCO Annual Meeting #ASCO19, May 31- June 4, 2019, Chicago, IL USA
References:
- Perner S, Demichelis F, Beroukhim R, et al. TMPRSS2: ERG fusion-associated deletions provide insight into the heterogeneity of prostate cancer. Cancer research 2006;66:8337-41.
- Tomlins SA, Laxman B, Varambally S, et al. Role of the TMPRSS2-ERG gene fusion in prostate cancer. Neoplasia 2008;10:177-IN9.
- Demichelis F, Fall K, Perner S, et al. TMPRSS2: ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene 2007;26:4596.
- Chinni SR, Semaan L, Cher M. TMPRSS2-ERG fusions confers efficacy of enzalutamide in an in vivo bone tumor growth model. AACR; 2018.
- Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in Metastatic Prostate Cancer before Chemotherapy. The New England journal of medicine 2014;371:424-33.


