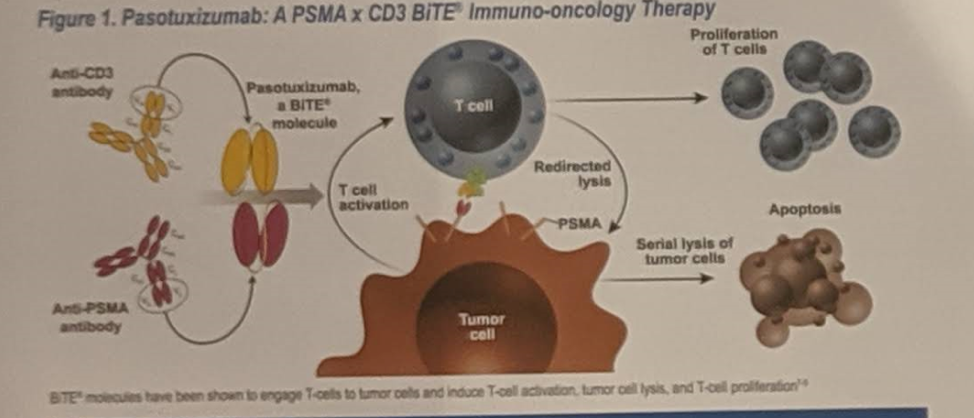
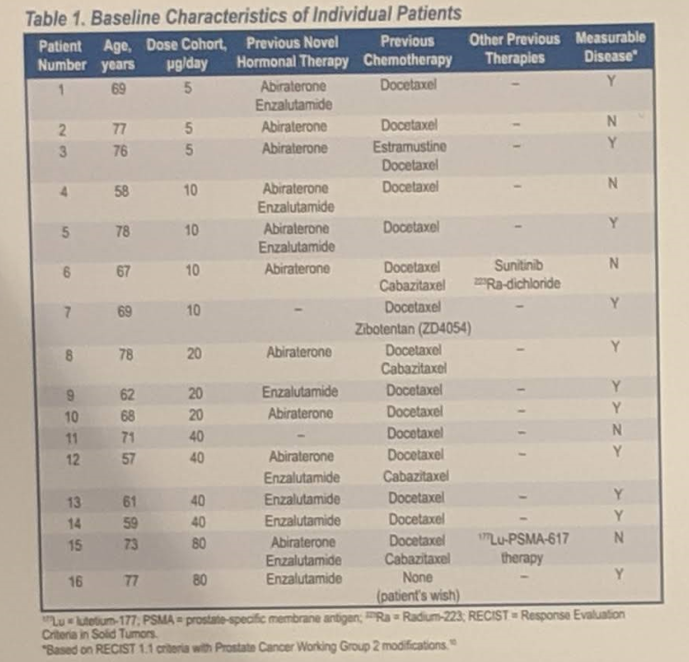

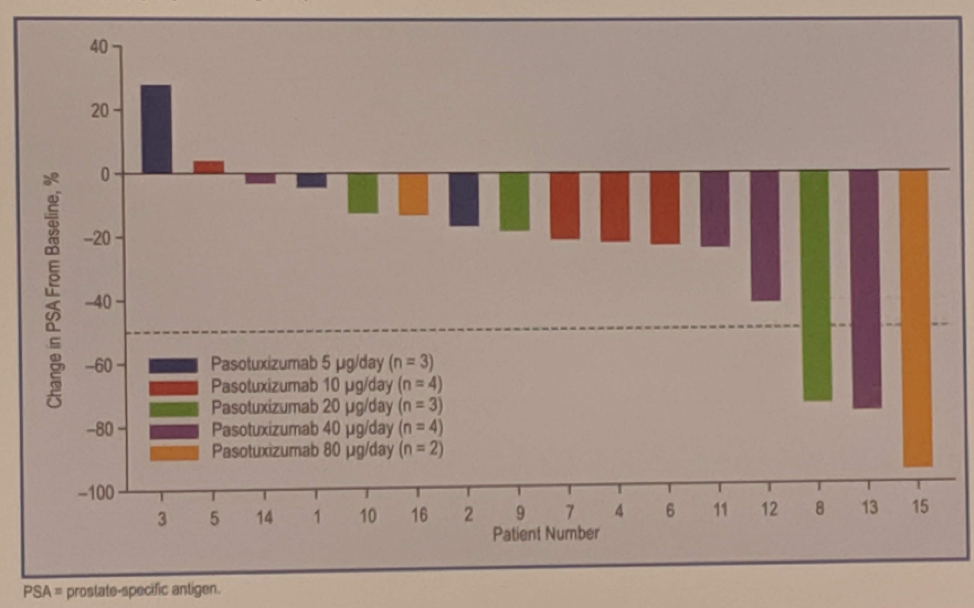
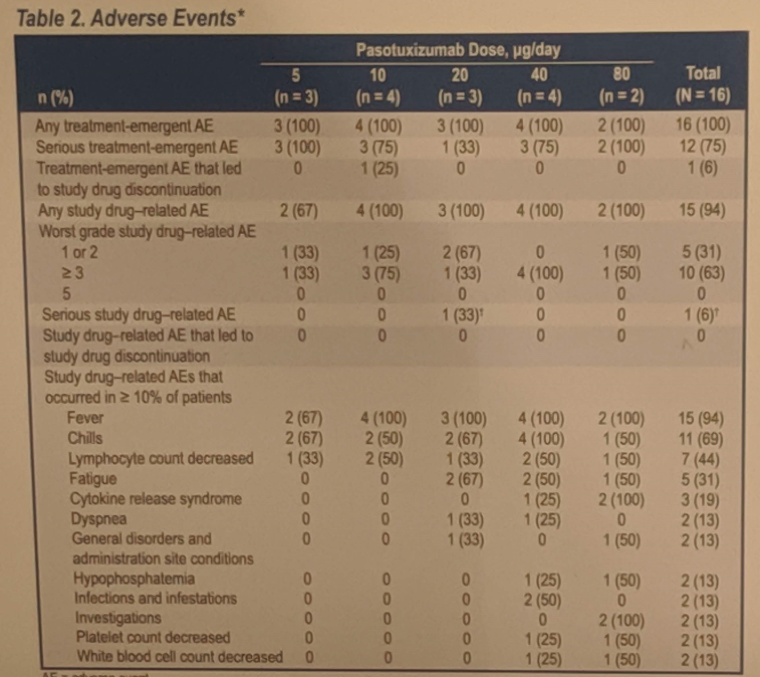
Pasotuxizumab is a prostate-specific membrane antigen (PSMA) targeting BiTE which was shown to be active against mCRPC in this study, with several patients experiencing a PSA50 and two patients with durable responses over one year. The side effect profile mimics the same symptoms as cytokine release syndrome which has been seen with other BiTEs used in hematologic malignancies, notably fever and chills which were experienced by the majority of patients. This study demonstrates that BiTEs may be another effective way to deliver an immunotherapy option for patients with mCRPC.
Presented by: Horst-Dieter Hummel, MD, University Hospital of Wuerzburg, Germany
Written by: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University, @TheRealJasonZhu at the 2019 ASCO Annual Meeting #ASCO19, May 31- June 4, 2019, Chicago, IL USA
References:
- Huehls AM, Coupet TA, Sentman CL. Bispecific T‐cell engagers for cancer immunotherapy. Immunology and cell biology 2015;93:290-6.
- Lutterbuese R, Friedrich M, Kischel R, et al. Preclinical characterization of MT112/BAY 2010112, a novel PSMA/CD3-bispecific BiTE antibody for the treatment of prostate cancer. AACR; Cancer Res 2011;71(8 Suppl): Abstract nr 4561.
- Friedrich M, Raum T, Lutterbuese R, et al. Regression of human prostate cancer xenografts in mice by AMG 212/BAY2010112, a novel PSMA/CD3-Bispecific BiTE antibody cross-reactive with non-human primate antigens. Molecular cancer therapeutics 2012;11:2664-73.


