This abstract provides data on 3057 men who had germline DNA testing done by Invitae in the United States. The majority of men were Caucasian (74%), but this study also provides data on a large cohort of African Americans as well (229, 7%), a population where there is a paucity of germline data. Also included in this analysis were 210 (7%) men who self-identified as Ashkenazi Jewish. Consistent with prior cohorts, 1.6% of men had the HOXB13 G84E gene variant which significantly increases one’s risk for developing prostate cancer. In terms of mismatch repair genes (MSH2/6, MLH1, PMS2, and MUTYH), there was no significant difference in the overall prevalence of pathogenic mutations between Caucasian Americans (CA), African Americans (AA), or Ashkenazi Jewish Americans (AJ) (CA 1.5% vs AA 0.9% vs AJ 1.4%, p = 0.89). There was also no difference in genes involved in homologous DNA repair (BRCA1/2, ATM, CHEK2, RAD51D, and PALB2) between the three cohorts of patients.
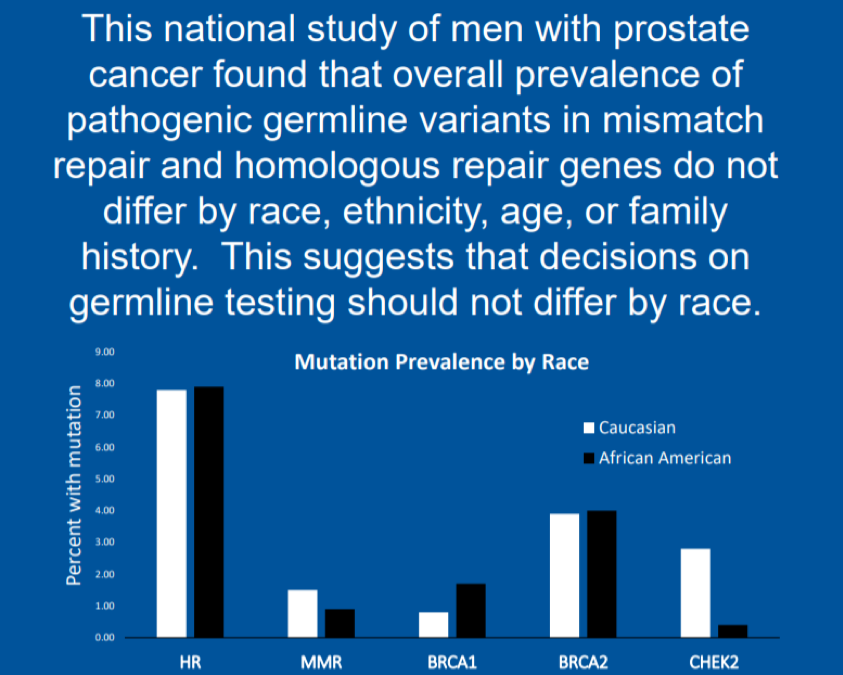
In this large retrospective analysis of over 3000 men with prostate cancer and germline testing, there was no significant difference in the overall prevalence of actionable germline mutations such as mutations in MMR (immunotherapy) or HR genes (PARP inhibitors). Age at the time of testing and self-reported family history of prostate cancer was also not associated with the presence of a germline mutation. In summary, this data suggests that all men with advanced prostate cancer should be offered germline testing, which is in line with the current NCCN guidelines.
Presented by: Alexandria Mara, MD, Internal Medicine Resident, Duke University School of Medicine, Durham, NC
Written by: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University, @TheRealJasonZhu at the 2019 ASCO Annual Meeting #ASCO19, May 31-June 4, 2019, Chicago, IL USA
References:
- Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and Heritable Factors in the Causation of Cancer — Analyses of Cohorts of Twins from Sweden, Denmark, and Finland. New England Journal of Medicine 2000;343:78-85.
- Hjelmborg JB, Scheike T, Holst K, et al. The Heritability of Prostate Cancer in the Nordic Twin Study of Cancer. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2014;23:2303-10.
- Mateo J, Carreira S, Sandhu S, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. New England Journal of Medicine 2015;373:1697-708.
- Ewing CM, Ray AM, Lange EM, et al. Germline mutations in HOXB13 and prostate-cancer risk. New England Journal of Medicine 2012;366:141-9.


