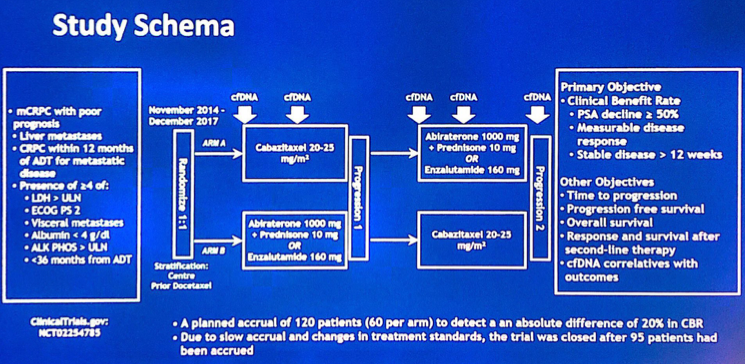
Poor prognosis for this trial was defined as: liver metastases, early CRPC (< 12 months from ADT start), and/or > 3 of 6 poor prognostic criteria 1. LDH > upper limit of normal, 2. ECOG PS2, 3. visceral metastasis, 4. albumin <4 g/dL, 5. alkaline phosphatase > upper limit of normal, 6. <36 months from ADT2 patients were randomized to receive cabazitaxel (Arm A) or androgen receptor targeted therapy (Arm B, abiraterone or enzalutamide by investigator choice), with cross over at progression. The primary objective was to determine the clinical benefit rate (defined as PSA decline ≥50% (PSA50), objective response, or stable disease ≥ 12 weeks). Plasma was sampled serially for circulating tumor DNA (ctDNA). The schema for the study is as follows:
 There were 95 patients randomized (Arm A: 45, Arm B: 50) of which 18% had liver metastasis, 88% had early CRPC and 30% had > 3 of 6 poor prognostic criteria. Among these patients, 52% had prior docetaxel, half for castration sensitive disease. Patients receiving cabazitaxel had a higher clinical benefit rate (88%) compared to patients receiving abiraterone or enzalutamide (70%; p = 0.043).
There were 95 patients randomized (Arm A: 45, Arm B: 50) of which 18% had liver metastasis, 88% had early CRPC and 30% had > 3 of 6 poor prognostic criteria. Among these patients, 52% had prior docetaxel, half for castration sensitive disease. Patients receiving cabazitaxel had a higher clinical benefit rate (88%) compared to patients receiving abiraterone or enzalutamide (70%; p = 0.043).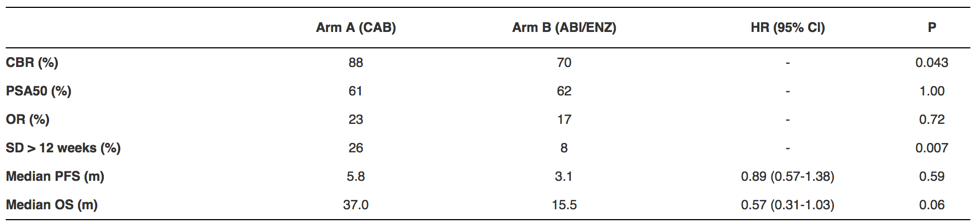
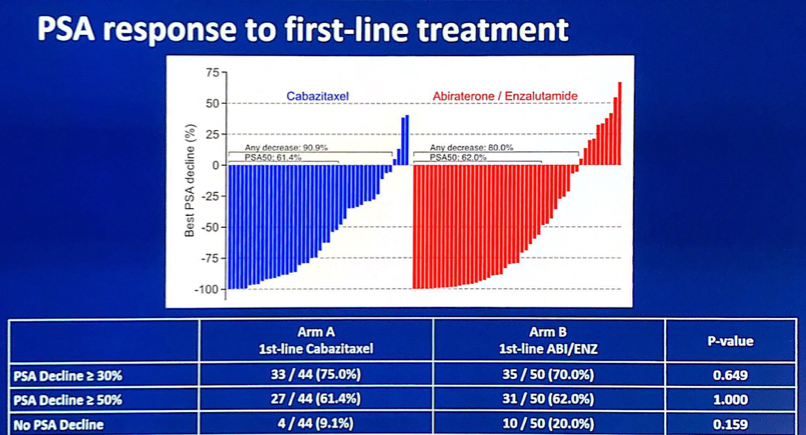
The PSA response to first-line treatment was comparable between the two arms:
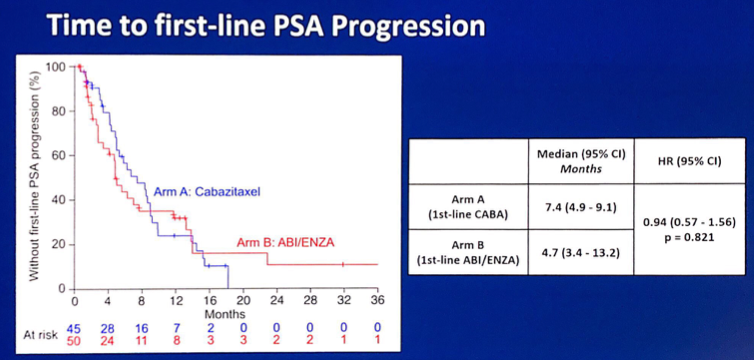
There was no difference between the arms for time to first-line PSA progression (HR 0.94, 95% CI 0.57-1.56):
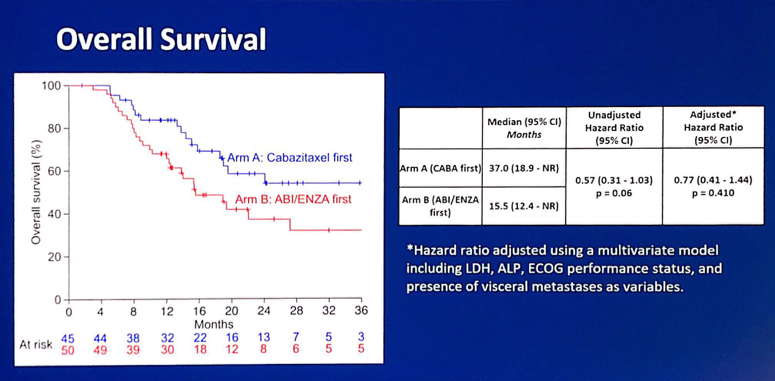
Furthermore, there was no difference in overall survival after adjusting in a multivariable model (HR 0.77, 95% CI 0.41-1.44):
Baseline ctDNA fraction > 15% (median) was associated with shorter 1st-line progression-free survival (median 2.8 vs 8.4 months, HR 2.54, p < 0.001) and overall survival (median 14.0 vs 38.7 months, HR 2.64, p = 0.001). Additionally, ctDNA alterations in AR, TP53, PI3K pathway, RB1, and DNA repair were detected in 53%, 45%, 31%, 23%, and 21% of patients, respectively. Shorter progression-free and overall survival were associated with androgen receptor gain (HR 2.57, 95% CI 1.63-4.06; HR 3.59, 95% CI1.9-6.69, respectively) and TP53 defects (HR 2.62, 95% CI 1.65-4.15; HR 3.33, 95% CI 1.8-6.14, respectively). Patients with concurrent defects in TP53 and RB1 had a trend for worse progression-free and overall survival than patients with TP53 defect alone. Finally, androgen receptor rearrangements predicted to disrupt the ligand binding domain were detected in 6% of patients and had shorter progression-free survival (HR 2.60, 95% CI 1.11 - 6.09) with a trend for shorter overall survival (HR 2.27, 95% CI 0.89 - 5.81).
Dr. Chi concluded his presentation of cabazitaxel versus abiraterone or enzalutamide for poor risk mCRPC with several points:
- There was a higher clinical benefit rate for cabazitaxel versus abiraterone or enzalutamide – a higher proportion of patients with stable disease in those treated with cabazitaxel
- Overall survival was not significantly different
- A strategy of using both cabazitaxel and androgen receptor pathway inhibitors as a treatment for poor prognosis mCRPC is appropriate
- Baseline and on-treatment ctDNA fraction were prognoses and genomic alterations were prognostic, particularly androgen receptor amplification and TP53 mutations
- In this population selected for poor prognosis, genomic alterations were not predictive for a differential treatment effect between cabazitaxel and androgen receptor inhibitors, however, the analysis is limited by small subgroups
Presented by: Kim Chi, MD, Medical Oncologist, British Columbia Cancer, Vancouver, BC, Canada
Co-Authors: Sinja Taavitsainen, Nayyer Iqbal, Cristiano Ferrario, Michael Ong, Deepa Wadhwa, Sebastien J. Hotte, Gregory Lo, Ben Tran, Arun Azad, Lori Wood, Joel Roger Gingerich, Scott A. North, Carmel Jo Pezaro, Joseph D. Ruether, Srikala S. Sridhar, Jack Bacon, Gillian Vandekerkhove, Matti Annala, Alexander William Wyatt; BC Cancer, Vancouver, BC, Canada; Urologic Sciences, University of British Columbia, Vancouver, BC, Canada; Saskatchewan Cancer Agency, Saskatoon, SK, Canada; Segal Cancer Centre, Jewish General Hospital, Montreal, QC, Canada; The Ottawa Hospital Cancer Centre, Ottawa, ON, Canada; BC Cancer Agency, Kelowna, BC, Canada; Juravinski Cancer Center, Hamilton, ON, Canada; R. S. McLaughlin Durham Regional Cancer Centre at Lakeridge Health, Oshawa, ON, Canada; Peter MacCallum Cancer Centre, Melbourne, Australia; School of Clinical Sciences, Monash University, Melbourne, Australia; Dalhousie University, Halifax, NS, Canada; University of Manitoba, Winnipeg, MB, Canada; University of Alberta Cross Cancer Institute, Edmonton, AB, Canada; Eastern Health Clinical School, Monash University, Melbourne, Australia; Tom Baker Cancer Centre, Calgary, AB, Canada; Princess Margaret Hospital, Toronto, ON, Canada; Vancouver Prostate Centre, University of British Columbia, Vancouver, BC, Canada; Vancouver Prostate Centre and Department of Urologic Sciences, Vancouver, BC, Canada
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia @zklaassen_md at the 2019 ASCO Annual Meeting #ASCO19, May 31-June 4, 2019, Chicago, IL USA
References:
- Gillessen S, Omin A, Attard G, et al. Management of patients with advanced prostate cancer: recommendations of the St. Gallen Advanced Prostate Cancer Consensus Conference (APCCC) 2015. Ann Oncol 2015;26(8):1589-1604.
- Chi KN, Kheoh T, Ryan CJ, et al. A prognostic index model for predicting overall survival in patients with metastatic castration-resistant prostate cancer treated with abiraterone acetate after docetaxel. Ann Oncol 2016;27(3):454-460.


