Pembrolizumab is a highly selective humanized monoclonal antibody that blocks the interaction between PD-1 and its ligands, PD-L1, and PD-L2. It currently has several indications for multiple types of solid tumors. The KEYNOTE-427 (Clinicaltrials.gov NCT02853344) is an open-label, multicenter, global phase 2 study (Figure 1) evaluating the efficacy and safety of pembrolizumab as a first line treatment for patients with advanced or metastatic RCC (Figure 1). It is divided to:
- Cohort A – showing the promising antitumor activity of a single agent pembrolizumab in previously untreated ccRCC4
- Cohort B – Assessed single agent pembrolizumab in previously untreated non-ccRCC patients

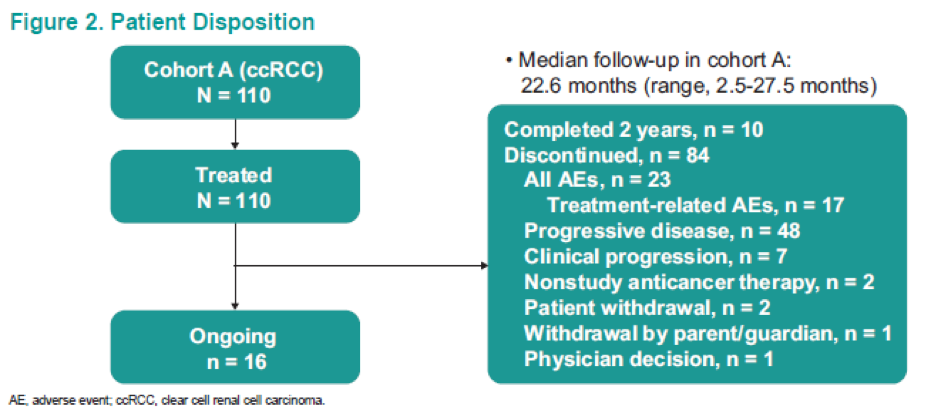
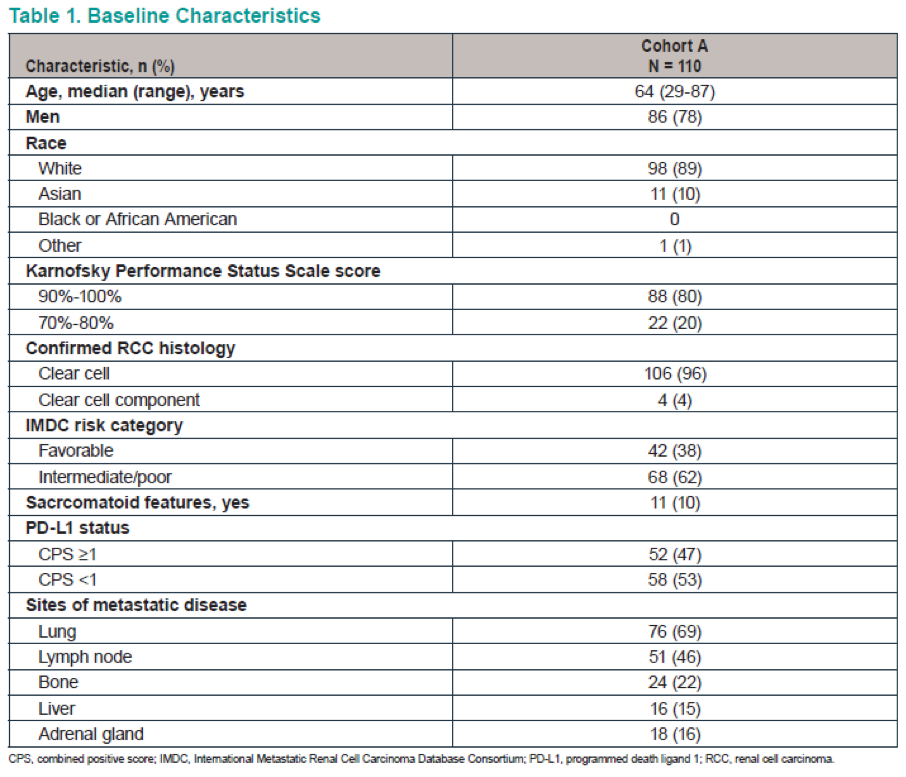
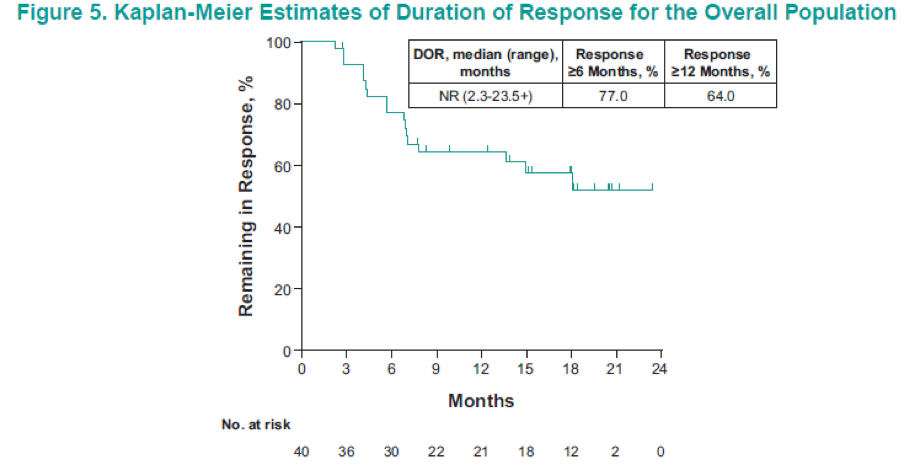
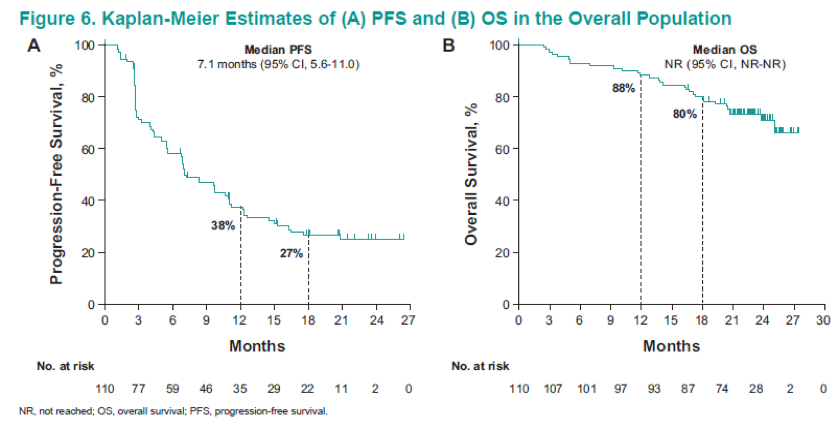
Figure 2 demonstrates the patient disposition, while table 1 shows the baseline characteristics of the 110 patients analyzed. Table 2 demonstrates the confirmed ORR in the overall population and the patient subgroups per RECIST v1.1. These results demonstrate an ORR of 36.4%, and a disease control rate of 57.3%. Figure 3 shows the maximum change from baseline in target lesions, with any decrease manifested in 68.2% of patients, and complete response of target lesions shown by 6.4%. Figure 4 shows the time to response and the duration of response in patients who had a confirmed complete or partial response. Figure 5 is a Kaplan-Meier curve, showing estimates of the duration of response for the overall population, with a median duration of response not reached. A total of 77% and 64% of patients responded for ≥ 6 months and ≥12 months, respectively. Table 3 shows the time to and duration of response in the overall population and the patient subgroups. The overall median time to response was 2.8 months, and the median duration of response was not reached. Lastly, figure 6 shows the PFS and OS in the overall population, with a median PFS of 7.1 months and a median OS that was not reached.




A total of 17 (15%) patients had to discontinue the drug because of treatment-related adverse events. Three patients died because of adverse events, and half of the patients were treated with concomitant steroids. The most common adverse effects were fatigue, pruritus, and diarrhea, each occurring in almost 30% of patients. A total of 29% of patients had grade 3-5 adverse effects. Immune-mediated adverse events occurred in 33% of patients, with hypothyroidism (14%), colitis (5%), hyperthyroidism (5%), and pneumonitis (5%) being the most common. A total of 15% had grade 3-5 immune-mediated adverse events.
The authors concluded that after a median of 23 months follow-up, first line pembrolizumab monotherapy continued to show promising antitumor activity in advanced ccRCC patients. The safety profile was comparable to that previously reported with pembrolizumab monotherapy.9 These results provide support for further exploration of pembrolizumab in the adjuvant setting (KEYNOTE-563, Clinicaltrials.gov NCT03142334), and inform outcomes with pembrolizumab combinations for advanced RCC.
Presented by: Scott S. Tykodi, MD, PhD, University of Washington and Fred Hutchinson Cancer Research Center, Seattle, WA
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre, @GoldbergHanan at the 2019 ASCO Annual Meeting #ASCO19, May 31- June 4, 2019, Chicago, IL USA
References:
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre, @GoldbergHanan at the 2019 ASCO Annual Meeting #ASCO19, May 31- June 4, 2019, Chicago, IL USA
References:
- Atkins MB et al. Immune checkpoint inhibitors in advanced renal cell carcinoma: experience to date and future directions. Ann Oncol Jul 2017 1;28(7):1484-1494.
- Motzer RJ et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med 2018 Apr 5;378(14):1277-1290.
- Motzer RJ et al. IMmotion 151: A randomized Phase III Study of Atezolizumab plus Bevacizumab vs. Sunitinib in Untreated Metastatic Renal Cell Carcinoma (mRCC). J Clin Oncol 2018;36(suppl 65; abstr 578)
- Rini BL et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019 March 21;380(12):116-1127.
- Lee CH et al. Levatinib + pembrolizumab in patients with renal cell carcinoma: Updated results. Journal of Clinical Oncology. 36, no. 15_suppl (May 20 2018) 4560-4560.
- Atkins MB et al. Pembrolizumab Plus Pegylated INterferon alfa-2b or Ipilimumab for Advanced Melanoma or Renal Cell Carcinoma: Dose-Finding Results from the Phase 1b KEYNOTE-029 Study. Clin Cancer Res 2018 Apr 15;24(8):1805-1815.
- Chowdhry S et al. Real-world outcomes in first-line treatment of metastatic castration-resistant prostate cancer (mCRPC): The prostate cancer registry. JCO 35, no. 6_suppl (February 20 2017) 212-212.
- Motzer RJ et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019
- Balar AV et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet Oncol 2017 Jan 7;389(10064):67-76.


