Rini et al.1 have previously demonstrated that active surveillance in a prospective phase 2 trial of 48 patients with treatment-naive, asymptomatic, metastatic renal-cell carcinoma, the median time on surveillance from registration on study until initiation of systemic therapy was 14.9 months. Based on this, and other data, surveillance is often utilized for low-volume mRCC disease in otherwise asymptomatic patients.
However, as the authors of this abstract note, few studies have assessed AS for mRCC compared to immediate treatment. As such, the authors aimed to assess the outcomes and safety of AS in comparison to immediate systemic treatment for mRCC pts. To address this, they utilized CKCis - Canadian Kidney Cancer information system. This is Canada’s largest database of patients with kidney cancer that collects prospective patient data from 14 Canadian academic centers.
The study includes mRCC patients diagnosed between January 1, 2011 and December 31, 2016. AS strategy was defined as: (1) start of systemic therapy ≥6 months after diagnosis of mRCC; or (2) never receiving systemic therapy for mRCC with overall survival (OS) ≥1 yr. This second point was used as a surrogate to exclude patients who were not started on treatment due to poor prognosis. Patients starting systemic treatment < 6 months after diagnosis of mRCC were defined as receiving immediate systemic treatment. Overall survival (OS) and time to failure of 1st line treatment failure (TTF) between the two cohorts were compared.
They identified a total of 863 patients who met criteria for AS (cohort A). Of these, 370 started treatment ≥ 6 months after their initial diagnosis (cohort A1) and 493 never received systemic treatment and were alive for ≥1 year (cohort A2). On the other hand, they identified 848 patients who received immediate systemic treatment (cohort B) – this was further broken down into B1 (within 3 months) and B2 (between 3-6 months). Group C were those who did not get systemic therapy and died within 1 year. Full demographics of the cohorts are seen below:
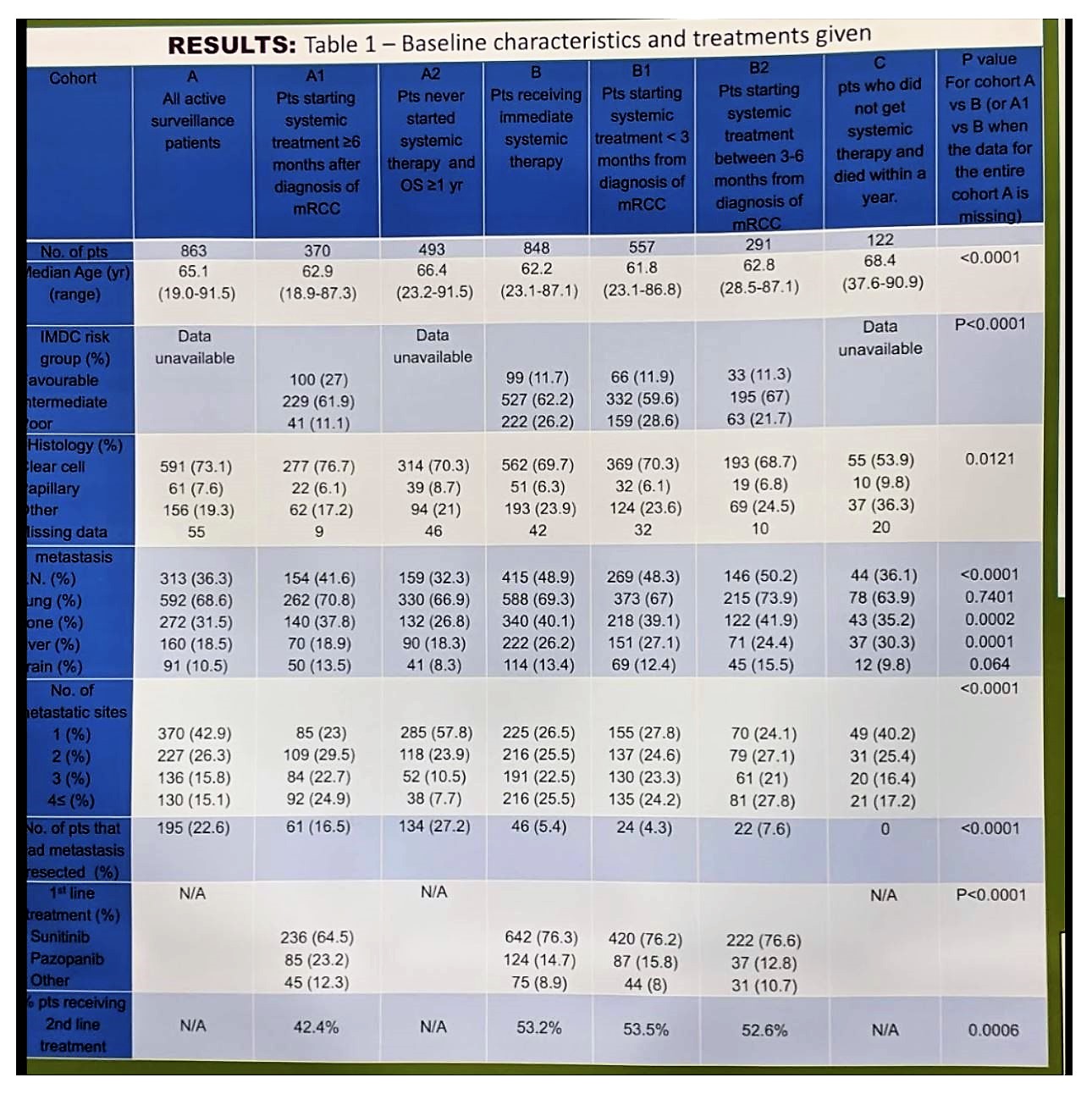
Five-year OS probability was significantly greater for cohort A than for cohort B (70.2% vs. 32.1%; P < 0.0001). After adjusting for IMDC risk criteria and age, both OS (HR 0.46, 0.38-0.56, P < 0.0001) and TTF (HR 0.79, 0.69-0.92, P = 0.0021) were greater in cohort A1 vs. B. This is seen below:
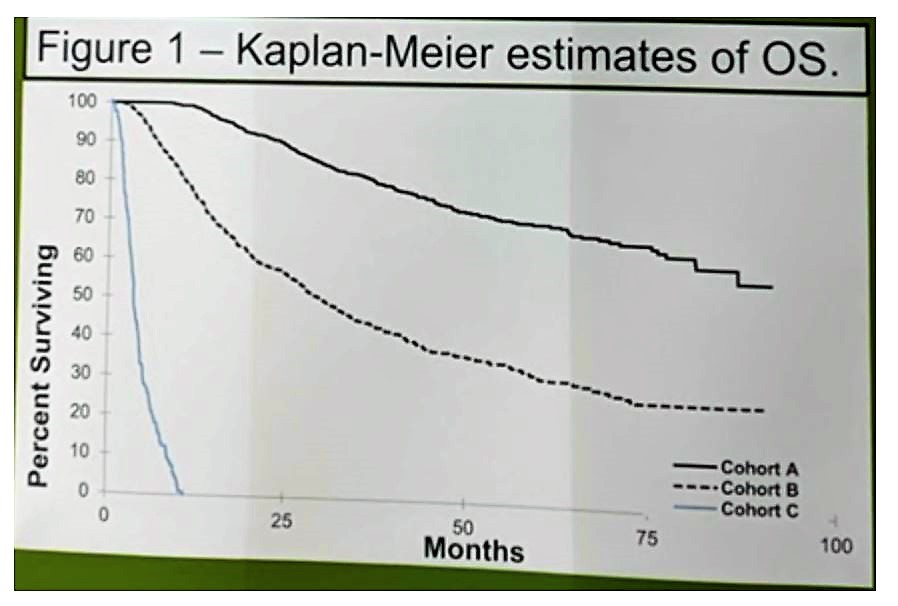
For cohort A1 the median time on AS was 14.2 m (range 6 – 71), very similar to the Rini paper.
Based on this study, which is the largest analysis of AS in mRCC to date, the data suggests that a subset of patients may be safely observed without immediate initiation of systemic therapy. They note, however, that as patients were treated between 2011 and 2016, a prospective validation is required in the contemporary immunotherapy era.
Presented by: Igal Kushnir, MD, Ottawa Hospital Cancer Centre, Ottawa, Ontario, Canada
Written by: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University, @TheRealJasonZhu, at the 2019 ASCO Annual Meeting #ASCO19, May 31- June 4, 2019, Chicago, IL USA
References:
- Rini BI, Dorff TB, Elson P, Rodriguez CS, Shepard D, Wood L, Humbert J, Pyle L, Wong YN, Finke JH, Rayman PA, Larkin JM, Garcia JA, Plimack ER. Active surveillance in metastatic renal-cell carcinoma: a prospective, phase 2 trial. Lancet Oncol. 2016 Sep;17(9):1317-24. doi: 10.1016/S1470-2045(16)30196-6. Epub 2016 Aug 3.


