This abstract provides data on 103 patients with a variety of different ADs such as psoriasis (22%), thyroiditis (20%), rheumatoid arthritis (13%), polymyalgia rheumatica (8%), inflammatory bowel disease (6%), multiple sclerosis (3%) and lupus (3%) who were treated with CPI in the first or second line setting.

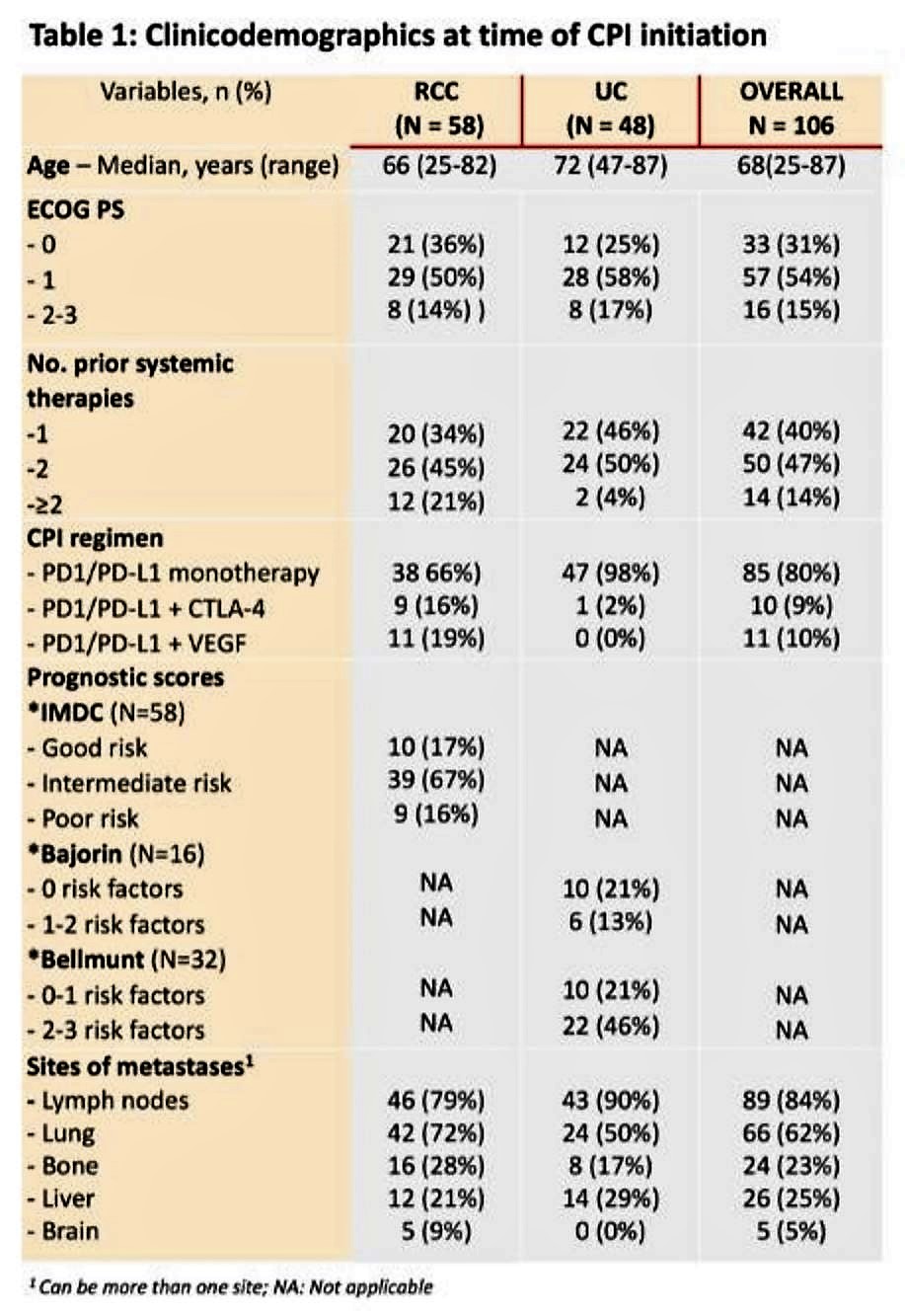
57 patients had RCC and 46 patients had UC and their baseline characteristics are shown below.
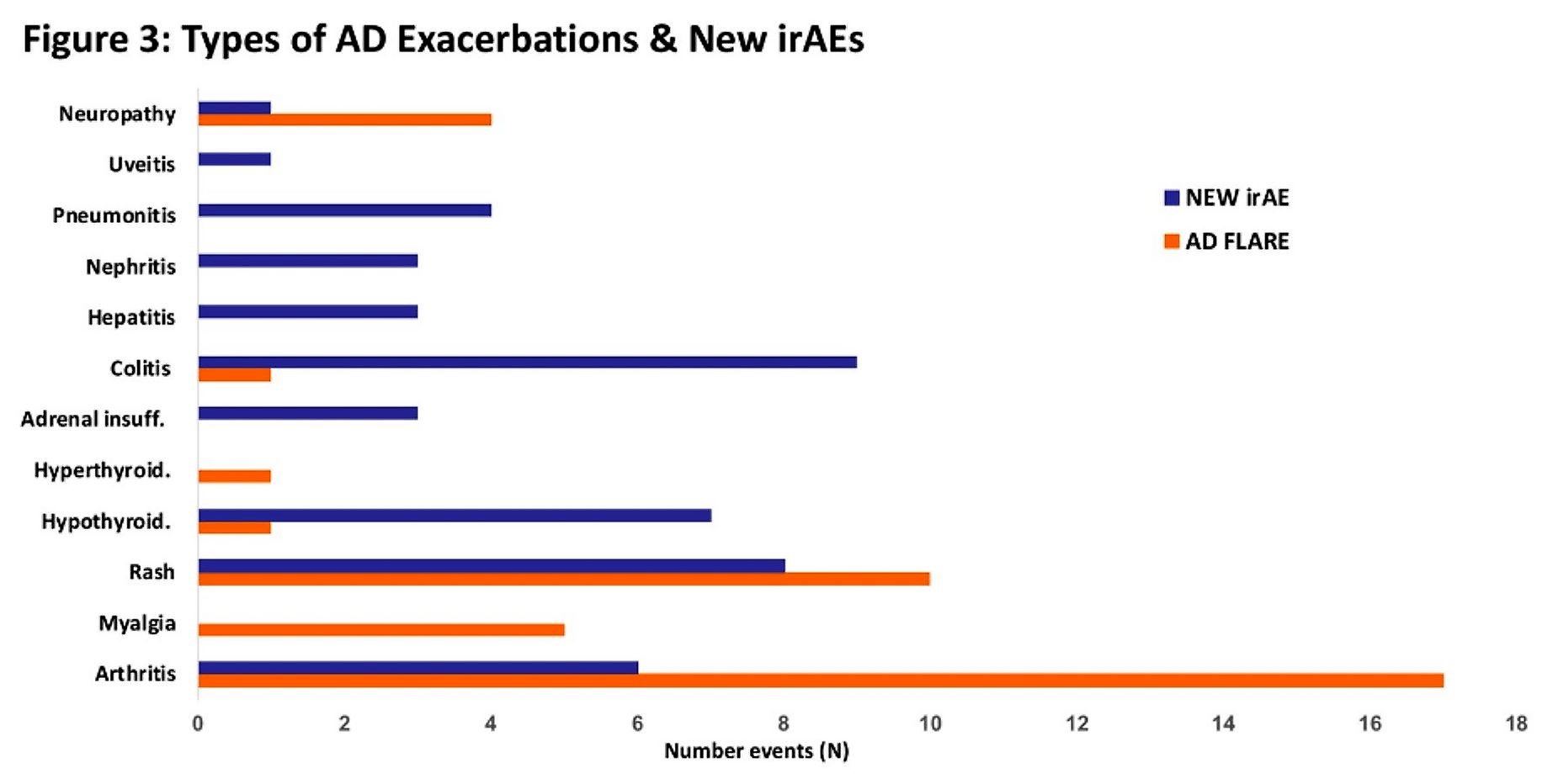
Prior to initiation of CPI therapy, 35% of patients had clinically active AD. 37% of patients experienced an exacerbation of their AD, most commonly arthritis and rash. Immune-related AEs unrelated to their ADs occurred in 36% of patients as well including colitis, rash, hypothyroidism, and nephritis.
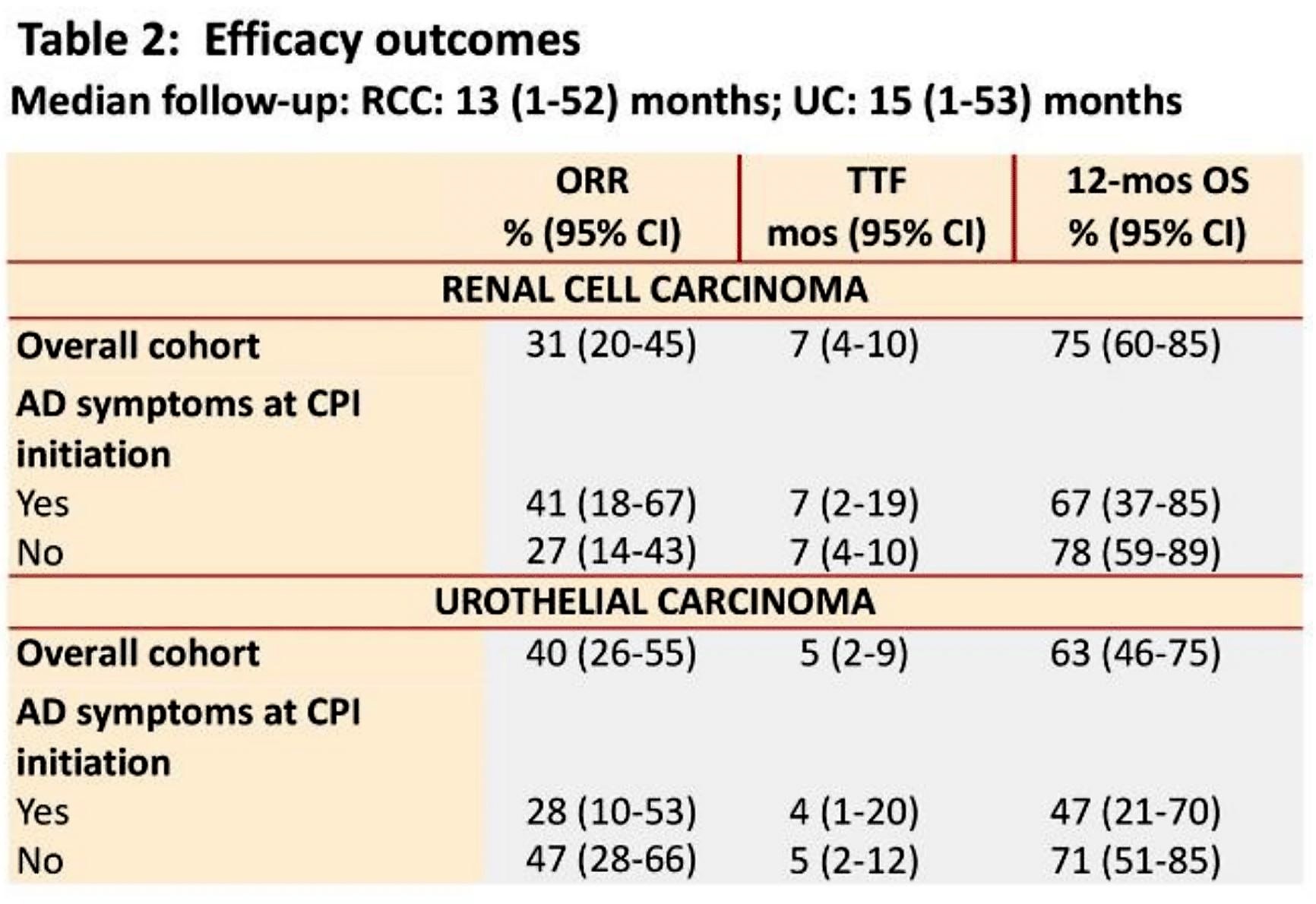
At the time of data cutoff, 16% of patients had discontinued CPI for toxicity. The ORR was 31% for RCC and 35% for UC, with a 1-year overall survival (OS) of 74% for RCC and 60% for UC.
This study sheds light on an important question for our patients with ADs and RCC/UC. While the study is limited by its size, this study shows that the rate of grade 3/4 toxicity amongst patients with ADs appears not too dissimilar than what was reported in where 19% of patients had a Grade 3/4 treatment-related adverse event. In this study, less than 10% of patients required discontinuation of therapy due to AD exacerbation. After careful counseling and control of preexisting ADs, CPIs may be safely given to patients with ADs.
Presented by: Nieves Martinez Chanza, MD, Jules Bordet Institute, Brussels, Belgium
Written by: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University, @TheRealJasonZhu, at the 2019 ASCO Annual Meeting #ASCO19, May 31- June 4, 2019, Chicago, IL USA
References:
- Johnson DB, Khushalani NI, Puzanov I, et al. Ipilimumab in metastatic melanoma patients with pre-existing autoimmune disorders. American Society of Clinical Oncology; 2015.
- Danlos F-X, Voisin A-L, Dyevre V, et al. Safety and efficacy of anti-programmed death 1 antibodies in patients with cancer and pre-existing autoimmune or inflammatory disease. European Journal of Cancer 2018;91:21-9.


