In this presentation, Terence Friedlander, MD, focused on combination therapies, and specifically on cancer antigen presentation, priming and activation, and targeting the microenvironment. He emphasized the fact that in the tumor’s immune evasion pathway, the use of PD-1 and PD-L1 inhibitors make up only a small part. There are opportunities in the rest of the cycle that can be targeted:
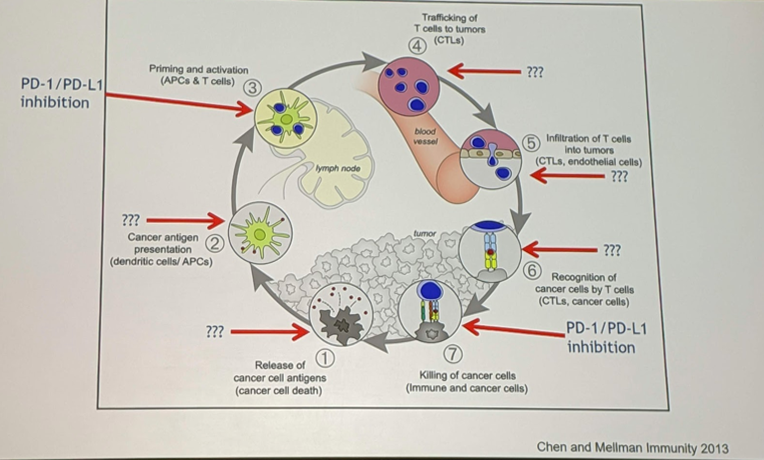
Cancer Antigen Presentation:
One method to help improve cancer antigen presentation is the use of vaccines. These, in theory, should work for bladder cancer, as bladder cancer has a high mutational burden (and, therefore, a higher number of neoantigens), but also a higher expression of self-antigens. There is also a relative abundance of cystectomy tissue to help identify these neoantigens.
There are 2 “off-the-shelf” vaccines, which means they are not personalized. These include:
- CV-301 – a recombinant vaccinia/fowlpox virus encoding for CEA and MUC1. Being assessed with atezolizumab in a phase 2 study of 68 patients.
- INO-5401 and IN0-9012 – plasmids encoding WT-1, PSMA, IL12, and hTERT. It is given with electroporation methods. This is being assessed with atezolizumab in a phase 2 study of 85 patients.
In a separate study, R07198457 is a rna-based vaccine being developed to be given in conjunction with atezolizumab. This is a phase 1a/1b trial assessing this combo in multiple malignancies, and urothelial is one of them.
Dr. Friedlander's pros and cons of this personalized vaccine approach are below:

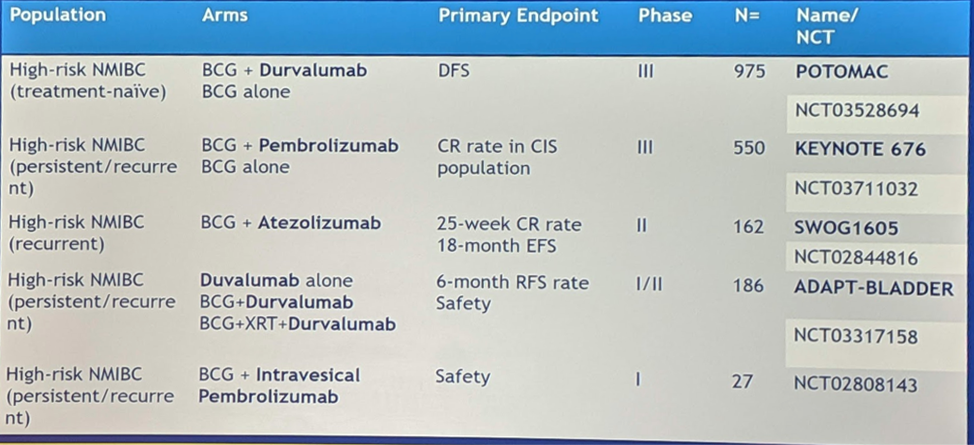
Then, Dr. Friedlander switched gears and addressed BCG. BCG is the original immunotherapy in cancer, having been utilized for urothelial carcinoma in non-muscle invasive bladder (NMIBC). In the NMIBC setting, there is though that co-administration with an ICI can generate more profound, potentially durable responses. Hence, there a 5 ongoing trials using BCG in combination with systemic ICI, or in one case, intravesical ICI:
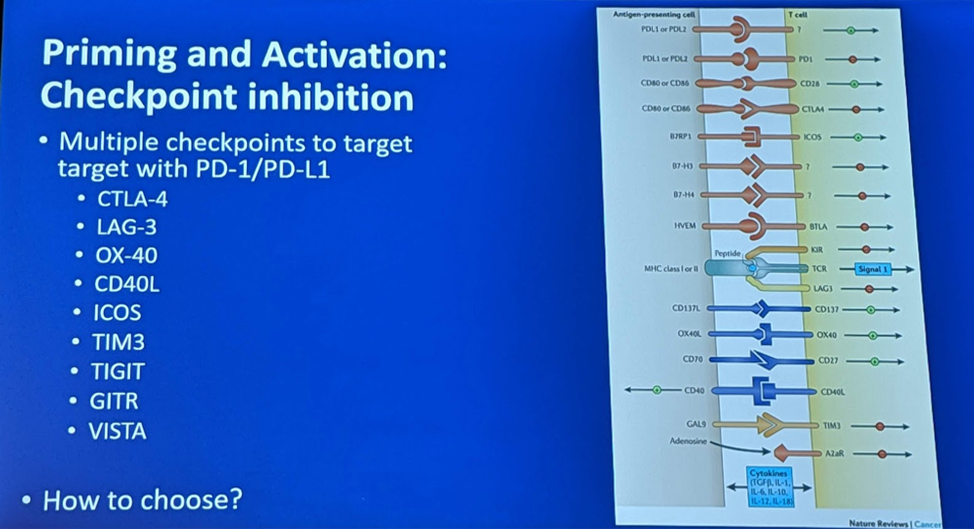
Priming and Activation:
The first agent discussed in this section is pegylated IL-2. IL-2 has a storied history, particularly in the treatment of metastatic renal cell carcinoma, but it was plagued by very significant adverse events – and required that patients were admitted to the hospital at the time of administration. IL-2 is a potent T-cell stimulant. However, more recently, it is experiencing a resurgence. Nektar Therapeutics had developed a pegylated IL-2 (NKTR-214), which allows for a slow release – and lower acute toxicity. But it is not yet clear if it has equivalent or better efficacy than IL-2. In the PIVOT-02 study2, 23 1st line cis-ineligible mUC patients were treated with NKTR-214 and nivolumab – they saw an objective response rate (ORR) of 48% with a 17% CR rate and 70% DCR, which is high. However, 18% grade 3 treatment-related effects were observed, which is higher than ICIs alone. Upcoming studies include: PIVOT 10 (randomized phase 2 trial of NKTR-214/nivo vs. gem/carbo in 1st line cis-ineligible mUC patients), PROPEL (NKTR-214 with atezo or pembro) and REVEAL (NKTR-214 + NKTR-262 +/- nivo).
He also mentioned that there are other targets for immune checkpoint inhibition besides the PD-1/PD-L1 and CTLA4 pathways. Others are seen below:
He briefly discussed some upcoming studies looking at the combination of CTLA-4 and PD-1/PD-L1 targeting, which has seen efficacy in mRCC management. In the Checkmate-032 study, the combination was tested in a phase 1/2 study of 274 platinum-pretreated patients. The response rates are seen below:

The combination of nivo 1 and ipi 3 had the best response of 38% ORR and 15 month overall survival (OS) benefit.
Durvalumab and tremelimumab are also being tested in combination, and in a phase 1 study had a 20% ORR. In the PD-L1 high population, ORR was 29% and the median OS was 19 months.
He highlighted the upcoming/ongoing phase III trials in this space:
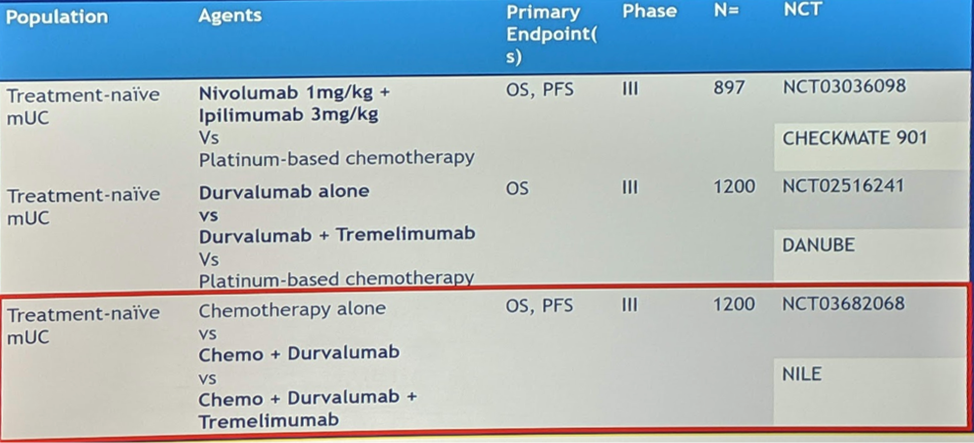
These will be large trials, based on the accrual numbers expected.
Targeting the microenvironment:
IDO – Indoleamine-2,3 dioxygenase – is an agent that depletes tryptophan in the tumor microenvironment, causes macrophage suppression and is an immunosuppressant. There are multiple inhibitors in development. Of note, anti-PD-L1 upregulates IDO expression, so there is a rationale for synergy.
Epacadostat was the initial agent in this space and had promising early results in combination with pembrolizumab. But in later melanoma trials, the results were disappointing, putting a temporary halt to further development. However, there is now renewed interest. Combining IDO + PD-1 is being done. BMS-986205 is being tested in combination with nivolumab in a phase 1 trial of mUC patients, and a 34% ORR with 1 CR was observed – this increased to 47% in PD-L1 positive patients. This combination is now under investigation in refractory NMIBC (4-arm randomized phase 2 trial – Nivo +/- BCG +/- BMS-986205). It is also being tested in MIBC in a 3-arm randomized phase III trial of 1200 patients (chemo, chemo + nivo + BMS-986205, chemo + nivo + placebo).
Presented by: Terence W. Friedlander, MD, Chief of Hematology-Oncology, Associate Director of the HDFCCC at Zuckerberg San Francisco General Hospital; Associate Clinical Professor, Division of Hematology/Oncology, University of California, San Francisco, San Francisco, CA
Written by: Thenappan Chandrasekar, MD, Clinical Instructor, Thomas Jefferson University, @tchandra_uromd, @JEFFUrology at the 2019 ASCO Annual Meeting #ASCO19, May 31- June 4, 2019, Chicago, IL USA
References:
- Grivas P, Drakaki A, Friedlander TW, Sonpavde G. Conceptual Framework for Therapeutic Development Beyond Anti-PD-1/PD-L1 in Urothelial Cancer. Am Soc Clin Oncol Educ Book. 2019 Jan;39:284-300. doi: 10.1200/EDBK_237449. Epub 2019 May 17. PMID: 31099684
- Siefer-Radtke GU ASCO 2019


